Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Carbon dioxide hydrogenated to methanol on large scale
Supported indium oxide catalyst could boost lab-scale process to an industrial level
by Stu Borman
March 25, 2016
| A version of this story appeared in
Volume 94, Issue 13

Manufacturers generally produce methanol, a key chemical building block and fuel, from petroleum-derived syngas, a mixture of carbon monoxide and hydrogen. Direct hydrogenation of the greenhouse gas carbon dioxide would be a more efficient and environmentally sustainable route to methanol. But practical catalysts capable of making this reaction happen on an industrial scale have been unavailable.
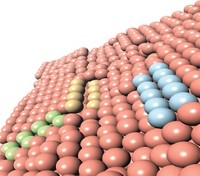
Scientists had shown earlier that indium oxide catalyzes the direct hydrogenation of CO2 to CH3OH on a lab scale. Javier Pérez-Ramírez of ETH Zurich and coworkers now demonstrate that zirconium oxide-supported In2O3 catalyzes the process under conditions similar to those required for industrial production (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201600943).
The supported catalyst can convert CO2 and H2 to CH3OH over at least 1,000 hours of continuous use and outperforms most other hydrogenation catalysts. The researchers proved experimentally that oxygen vacancies on the catalyst surface make the reaction possible—a mechanism predicted by theoretical calculations from a team led by Qingfeng Ge of Southern Illinois University and Tianjin University (ACS Catal. 2013, DOI: 10.1021/cs400132a).
The ETH Zurich group optimized the reaction by adding CO to the starting materials and varying the temperature, both of which tuned the number of vacancies. The technique is “a long-sought breakthrough with the potential to realize continuous CO2 conversion to methanol on a commercial scale,” Ge says.
Pérez-Ramírez and coworkers have filed patent applications on the technology in collaboration with French energy firm Total, which has started pilot studies of the process.
This article has been translated into Chinese and can be found here.
To see all of C&EN’s articles that have been translated into Chinese, visit http://cen.acs.org/cn.html.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter