Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
New fate for atmospheric nitric acid
Photochemical reactions could lead to source of ozone-forming nitrogen oxides
by Celia Henry Arnaud
April 13, 2016
| A version of this story appeared in
Volume 94, Issue 16
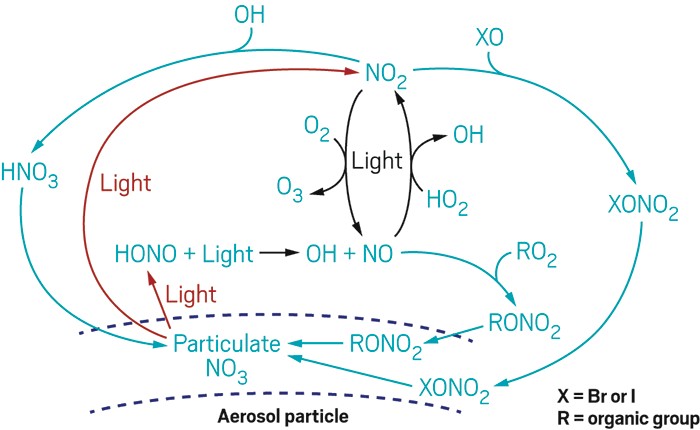
Because nitric acid is stable, long-lived, and not photochemically reactive, it’s long been thought to be the final destination for reactive nitrogen species in the atmosphere. But those assumptions might be wrong.
A team led by Xianliang Zhou of the New York State Department of Health and the State University of New York in Albany proposes that nitric acid in the atmosphere gets quickly recycled back into nitrous acid (HONO) and other forms of nitrogen oxides (NOx) via photochemical reactions involving nitrate in aerosol particles (Nature 2016, DOI: 10.1038/nature17195).
Nitrogen oxides are involved in the formation of ozone in the atmosphere. The findings could lead to atmospheric chemistry models with improved estimates of nitrogen species and ozone.
In the study, Zhou and coworkers performed airborne and lab-based measurements of samples from the marine boundary layer, the portion of the atmosphere in direct contact with the ocean. In field measurements obtained during flights over the North Atlantic Ocean, the researchers detected HONO concentrations of about 10 parts per trillion. That may not sound like a lot, but HONO rapidly undergoes photochemical reactions so there must be some source producing the acid, Zhou says.
In those field measurements, the team found no correlation between HONO and NOx, a possible precursor of HONO. Instead, they found a correlation between the concentrations of HONO and nitrate in aerosol particles.
To test whether HONO could indeed come from particulate nitrates, the team ran photochemical experiments with aerosol samples back in the lab.
“Our results show there’s a rapid photochemical recycling process that converts nitric acid and particulate nitrate into more reactive species like HONO and NOx,” Zhou says. “If you’re over land, you have many NOx sources, so the recycling is not as dominant. But if you go to a remote area where the emission source is really low, then the recycling process becomes more obvious and more important.”
“Measurements of NOx that my group has been making over the past 10 years have been consistently higher than those that are predicted by models,” says James Lee, an atmospheric chemist at the University of York, who makes atmospheric measurements in remote parts of the Atlantic. “This recycling mechanism has the potential to reconcile this.” He says this finding is important because increased NOx in remote areas could signal more ozone production.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter