Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Turning on bioorthogonal reactions catalytically
Catalysts could help researchers control when and where tetrazine ligations occur in cells or organisms
by Stu Borman
April 29, 2016
| A version of this story appeared in
Volume 94, Issue 18

To attach probes for imaging to biomolecules inside cells or organisms, chemists have developed so-called bioorthogonal reactions that don’t interfere with the biochemistry of the living things. Tetrazine ligation is the fastest of these bioorthogonal reactions, and its speed allows scientists to use small amounts of reagents to get the job done.
A new catalytic form of this ligation could enable researchers to control the timing and location of the reaction inside cells or organisms by turning on the chemistry with either an enzyme or a pulse of light.
Early bioorthogonal reactions were slow, so scientists had to introduce reagents at undesirably high concentrations to ensure the compounds could react before being cleared away by metabolic processes.
Researchers developed faster reactions so they could use reagents at lower concentrations. Tetrazine ligation, which is now used widely in bioorthogonal chemistry, involves the cycloaddition of tetrazine with a trans-cyclooctene or another strained alkene conjugated with a biomolecule.
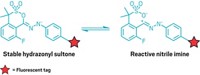
Joseph Fox and his coworkers at the University of Delaware helped develop tetrazine ligation and have now enhanced the technique by finding ways to turn it on catalytically (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b02168). The scientists use an enzyme or a near-infrared light-induced photocatalyst, methylene blue, to convert unreactive dihydrotetrazine to reaction-ready tetrazine (shown). Researchers have used ultraviolet light to activate bioorthogonal reactions before, but near-IR radiation is less damaging to tissue and penetrates more deeply than UV, about 0.6 cm.
“Numerous colleagues have asked me if there are good ways to use light to spatiotemporally control tetrazine ligations,” and now there is, comments Neal Devaraj of the University of California, San Diego, another developer of the tetrazine ligation technique.
“This is an exciting development,” says bioorthogonal chemistry specialist Qing Lin of the University at Buffalo, SUNY. “Potential applications include cell imaging, prodrug activation, and tissue engineering.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter