Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Asymmetric third ring is a charm for indole-annulated compounds
Chemists develop an enantioselective synthesis for constructing medium-sized rings on indole’s two-ring framework
by Stephen K. Ritter
May 9, 2016
| A version of this story appeared in
Volume 94, Issue 19
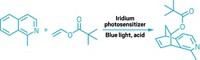
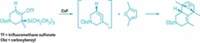
Fused-ring systems are special structural features of many natural product and synthetic compounds, contributing to the bioactivity that makes them useful as pharmaceuticals and agrochemicals. Chemists have numerous methods for generating small rings with six or fewer members, such as Diels-Alder and other cycloaddition reactions. They also have ways of constructing large rings with 10 or more members, including macrolactonization and ring-closing metathesis. However, the direct synthesis of medium-sized rings, especially enantioselectively, has remained a challenge because of steric constraints. Lin Huang, Li-Xin Dai, and Shu-Li You of Shanghai Institute of Organic Chemistry have now discovered a pathway around this roadblock by developing an intramolecular cascade reaction for building asymmetric seven- to nine-membered rings onto indole frameworks (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b02678). As an example, the team found that a chiral iridium catalyst can drive dearomatization of an allylic carboline to form a bridged intermediate. A subsequent ring-opening retro-Mannich step followed by hydrolysis results in expanding the size of the piperidine ring (shown). The researchers anticipate that their method will facilitate synthesis of new indole-annulated compounds to join those already among the ranks of commercial products.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter