Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
A nanowire battery that won’t die
Gel electrolyte dramatically extends nanowire electrode lifetime to 100,000 charges
by Katherine Bourzac
May 9, 2016
| A version of this story appeared in
Volume 94, Issue 19
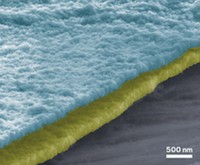
Imagine a battery that never stops holding a charge. An experimental energy storage electrode lasts as long as researchers have had the patience to charge and recharge it—so far, more than 100,000 times (ACS Energy Lett. 2016, DOI: 10.1021/acsenergylett.6b00029). Nanowire battery electrodes can provide a lot of power in a small footprint, says Reginald M. Penner of the University of California, Irvine, but they are prone to breaking. Penner’s group used a test setup to study manganese dioxide-coated gold nanowires, a potential lithium-ion battery cathode material. The group tested the wires with a typical liquid electrolyte and with a polymethyl methacrylate (PMMA) gel electrolyte—commonly used in solid-state batteries. Cathodes paired with the liquid electrolyte survived 2,000 to 8,000 charge cycles, but the one with PMMA survived 100,000 full charges and discharges—even in a test system designed to encourage failure. The gel may hold the MnO2 in place, Penner says, preventing it from breaking off of the gold below. Or the gel may slowly leak into pores in the MnO2 and plasticize it, preventing fracturing, he suggests.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter