Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemists reversibly evolve enzyme
Demonstration shows that structural dynamics play an important role in evolving new enzyme functions
by Celia Henry Arnaud
September 14, 2016
| A version of this story appeared in
Volume 94, Issue 37

Protein motions and structural dynamics play an important role in the evolution of new enzyme functions, an international team reports.
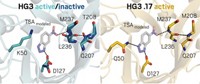
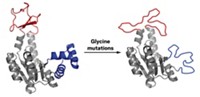
To explore the importance of protein dynamics, Nobuhiko Tokuriki of the University of British Columbia, Colin J. Jackson of Australian National University, and coworkers started with an enzyme called a phosphotriesterase that normally hydrolyzes organophosphates. They then “evolved” this enzyme into an arylesterase, one that instead hydrolyzes aromatic esters, by doing multiple rounds of directed evolution. Finally, they turned around and evolved that arylesterase back into a phosphotriesterase, analyzing the crystal structures and dynamics of multiple proteins along both the forward and reverse evolutionary trajectories (Nat. Chem. Biol. 2016, DOI: 10.1038/nchembio.2175).
Undertaking the process in both directions helps elucidate the connection between protein sequence, structure, and function.
The team’s analysis revealed that major changes in enzyme activity could be achieved by mutating amino acids remote to the active site. “Despite huge changes in activities—several orders of magnitude—there was really only one mutation directly in the active site,” Jackson says. “We don’t necessarily need to completely change the active site to achieve very large changes in function.”
Instead, remote mutations change interactions such as salt bridges and hydrogen bonds significantly over the evolutionary pathways. Along the trajectories, active site mutations occurred, followed by other mutations that helped optimize the positioning of the residues in the new active sites. That optimization involved changes in the motions of some loops in the proteins.
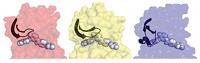
Some of the enzymes that the researchers identified along the trajectories could catalyze both reactions equally well and better than some naturally occurring enzymes for the same reactions. This bifunctionality seems to stem from the proteins’ flexibility, Jackson says.
But there’s a selective pressure during the evolution process to freeze out unnecessary motions by reducing that flexibility. “The mobility isn’t necessarily a positive thing for catalysis,” Jackson says. As the enzymes become more specialized, they also become less flexible in some regions.
The new study successfully linked the evolution of enzyme function to changing protein dynamics, says Joelle Pelletier, a chemistry professor at the University of Montreal who uses directed evolution to study protein catalysis. “Some regions adopt new wiggle-room to test out conformations, later stabilizing the most productive conformations with further evolution, while another switches from dynamic to rigid. That fine-tuning allowed specialization for one function to the detriment of the other.”
The researchers hope that their findings will be incorporated into methods for rationally designing proteins. “The obvious challenge is to take what we think we have learned and try to apply it rationally,” Jackson says. “If we can’t make things rationally, we’re still missing something.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter