Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Two-Step Delivery Method Ups Cancer Drug’s Concentration In Tumors
Drug Delivery: Administering empty nanoparticles followed by a cancer drug could be a more effective therapy than giving the drug alone
by Stu Borman
January 20, 2016
| A version of this story appeared in
Volume 94, Issue 4

Researchers report that a two-step procedure—delivering a tumor-localizing, drug-absorbing nanoparticle followed by the actual therapeutic—increases the amount of drug that reaches tumor cells and the time the drug acts on cells (Sci. Rep. 2016, DOI: 10.1038/srep18720).
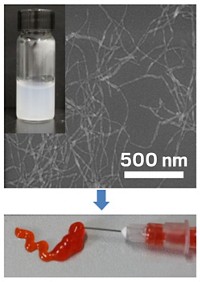
Mark W. Grinstaff of Boston University, Yolonda Colson of Brigham & Women’s Hospital, and coworkers first administered a polymer-based nanoparticle to mice with tumors. This nanoparticle swells and becomes a gel in mildly acidic surroundings. Because cancer cells are more acidic than normal cells, the tiny particles expanded to 10 times their original size inside tumors and became trapped.
Two days later, the researchers gave the mice Taxol (paclitaxel), which concentrated inside the trapped nanoparticles because of its hydrophobicity. Taxol concentrations within the mouse tumors rose to about five times the level attained when researchers gave mice the drug alone. The drug also remained in the tumor longer, giving Taxol more time to kill cancer cells.
When Grinstaff, Colson, and coworkers delivered nanoparticles preloaded with Taxol to mice with cancer, orders of magnitude more drug got to the tumors than was achieved with the two-step procedure. This might be expected because the drug doesn’t have to find the nanoparticles in the body.
But the new approach has an advantage: Nanomedicines preloaded with drugs would be considered new drug entities by the Food & Drug Administration and would have to pass a full series of clinical trials. Empty nanoparticles, on the other hand, are defined by FDA as medical devices because they do not have chemistry-based therapeutic activity. Taxol is also already FDA-approved. So the two-step strategy could minimize drug approval complications and expenses.
Cancer nanomedicine expert Joseph M. DeSimone of the University of North Carolina, Chapel Hill, notes that the study “reports a first-of-its-kind result” because the two-step treatment requires neither selective binding interactions between drug and nanoparticle nor an activating event to make the drug start working, either or both of which were required in earlier drug-nanoparticle treatments.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter