Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluoroamide fluorinates itself
In an iron-catalyzed process, the reagent acts as the fluorine source and provides its own directing group for late-stage C–H fluorinations
by Stephen K. Ritter
October 10, 2016
| A version of this story appeared in
Volume 94, Issue 40
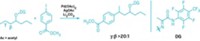
When it comes to fluorination of C–H bonds, chemists typically select one reagent to supply the fluorine and must decide whether the target substrate molecule requires a directing group or not to help activate the desired C–H bond and point the fluorine in the right direction. Brian J. Groendyke, Deyaa I. AbuSalim, and Silas P. Cook of Indiana University, Bloomington, have reduced the number of decisions to be made by developing a fluoroamide reagent that acts as the fluorine source and provides its own directing group to fluorinate itself (J. Am. Chem. Soc. 2016, DOI: 10.1021/jacs.6b08171). Undirected C–H fluorinations generally are catalyzed radical reactions that don’t provide optimal C–H bond selectivity, the researchers point out. Directed approaches offer precise targeting, but they remain limited in the types of functionalized molecules that can be used as the substrates and typically require a costly palladium catalyst. The researchers found they could overcome those limitations with fluoroamides made by simply treating a parent amide with N-fluorobenzenesulfonimide. Adding low-cost iron triflate to functionalized benzylic fluoroamides promotes iron-mediated fluorine transfer under mild conditions to make fluorinated benzylic amides. The team suggests this new method could be beneficial in selectively fluorinating molecules during the late stages of complicated pharmaceutical syntheses.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter