Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Fluorinated azides click to make triazoles
Researchers create new reagents for a fluorinated version of copper-catalyzed azide-alkyne click chemistry
by Stephen K. Ritter
December 15, 2016
| A version of this story appeared in
Volume 94, Issue 49
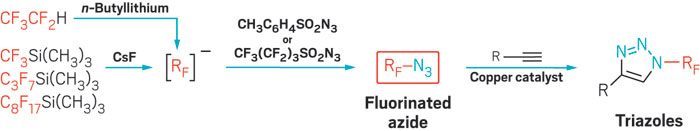
With the help of new fluorinating reagents, a team led by Zsófia E. Blastik and Petr Beier of the Czech Academy of Sciences has devised a versatile fluorinated version of copper-catalyzed click chemistry. The approach overcomes some previous difficulties with preparing suitable fluorinated reagents for click reactions and opens the door to broader use of click chemistry, which has become invaluable to chemical biology and materials science.
The new chemistry hinges on the ability to make azidoperfluoroalkanes. Azidotrifluoromethane (CF3N3) has been known for some time, but its best reported synthesis starting from CF3I requires cumbersome handling of toxic and corrosive CF3NO, N2H4, and Cl2 gases—an approach that has limited CF3N3’s applications. Beier’s group alternatively attempted using electrophilic CF3I with sodium azide (NaN3) as a nucleophile, but the reaction didn’t work. Instead, the team found that CF3N3 and its previously unknown longer chain analogs can be prepared more conveniently from CF3Si(CH3)3 and related nucleophiles and sulfonyl azide electrophiles. In effect, the researchers flipped the polarity of the reaction’s constituents (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201609715).
The reported azidoperfluoroalkanes undergo copper-catalyzed azide-alkyne cycloadditions, also known as click reactions, leading to N-perfluoroalkyl triazoles as underexplored building blocks, Beier says. “In fact, azidoperfluoroalkanes are more reactive in the click reaction with alkynes than nonfluorinated alkyl azides.”
“A reminder emerging out of this paper by Beier and colleagues is that, if you want to build a bond by a polar mechanism, there are always two options,” says Andrei K. Yudin of the University of Toronto, who focuses on the chemistry of heterocyclic compounds. “It is a good idea to reverse the polarity of components if one path is problematic. As a result, they have developed an efficient entry into azidoperfluoroalkanes.”
CORRECTION: On Jan. 9, 2017, this story was updated to correct one of the starting reagents in the reaction scheme. It should be C8F17Si(CH3)3 rather than C8H17Si(CH3)3 as originally stated.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter