Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Chemists Confirm The Identity Of Pivotal Intermediate In Carbocation Rearrangements
Reaction mechanisms: Computational study solves a long-standing chemical conundrum on alkane branching
by Stephen K. Ritter
February 15, 2016
| A version of this story appeared in
Volume 94, Issue 7
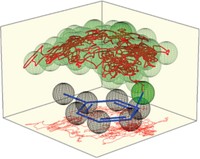
Chemists have known for 70 years that carbocation rearrangements involve slow steps and fast steps. The slow steps are crucial in that they control the degree of branching in alkanes, and understanding how that works is vital for predicting product distributions in processes such as biomass and petroleum refining. But why the key steps are slow has been a confounding mystery. Daniel J. S. Sandbeck, Daniel J. Markewich, and Allan L. L. East of the University of Regina now appear to have found the answer via a set of computer simulations (J. Org. Chem. 2016, DOI: 10.1021/acs.joc.5b02553). The team first revisited decades-old studies in which chemists proposed that the rearrangements proceed through a protonated cyclopropane intermediate. Then using hexyl ion as a model, the researchers ran simulations and uncovered 70 transition states connecting primary, secondary, and tertiary ion versions. In the past, most chemists thought the cyclopropane intermediate must be protonated on an edge of the ring or at one of the corners. The Regina researchers found that neither assumption was correct but that the intermediate takes on a mesomeric structure that is a hybrid of the two. The finding suggests that branching is slow because the reaction pathway must pass through an unstable primary carbocation that involves the mesomeric structure.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter