Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Going to new lengths with better fibers
Microfluidics produces thinner, stronger, longer collagen fibers
by Neil Savage
September 23, 2016

Being able to replace torn tendons or repair damaged nerves is an attractive prospect in medicine, and it will probably require being able to produce collagen fibers that mimic the real thing. Unfortunately, existing methods for making collagen fiber don’t reach that goal. The main method, wet spinning, produces fibers that are relatively short and a bit too thick. Now a team of researchers has used microfluidics to create fibers that are thinner, stronger, and virtually any length (Nano Lett. 2016, DOI: 10.1021/acs.nanolett.6b02828).
“We really could make hundreds of meters,” says Thomas Scheibel, a professor of bioengineering at the University of Bayreuth.
Microfluidic devices, which can mix reagents and allow them to react while flowing through microscopic channels, have been used to create other fibers, such as alginate and silk. For collagen, though, they’ve so far only been useful for making short fibrils. Collagen fibers have a triple-helix structure made of individual collagen polypeptides. The fibers self-assemble from dissolved collagen at basic pH when flow conditions help align the strands with each other. Longer fibers result from stronger fibers, which result when the strands assemble rapidly and when alignment is good. Part of the problem is that it can take hours for the collagen fibers to assemble and gel, too long for the process to be practical. And the fibrils can stick to the walls of the channel and block it.
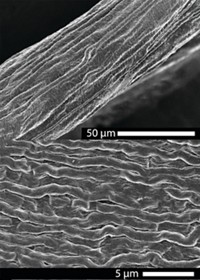
In the new approach, the team dissolved commercially available collagen type I—the most abundant type of collagen in the body—in acetic acid and flowed the solution through a 200 by 210 µm microfluidic channel. To prevent sticking, the team designed their microfluidic device so that a buffer solution came in through two inlets and completely surrounded the collagen-bearing solution, preventing it from touching the walls. The buffer created a slightly basic environment at the interface with the collagen solution, triggering the dissolved collagen to form fibrils. Polyethylene glycol in the surrounding buffer also pulled water out of the collagen solution, causing the fibers to precipitate much faster than in previous processes. The hydrodynamic forces of the mixture flowing through the channel caused the fibrils to align and assemble into microfibers, which the team collected and spun around a spool as they exited the channel.
The better the triple helix structure is aligned, the stiffer and stronger the resulting fiber is. The team’s method led to smaller-diameter fibers that had better aligned units and fewer defects, producing fibers that were approximately three times as stiff and twice as strong as wet-spun fibers. This strength allows the fibers to be grown to any length. By altering the buffer flow rate, the team produced fibers from 3 to 6 µm in diameter, whereas wet spinning produces fibers no smaller than 8 µm in diameter.
Scheibel says being able to vary the diameter of the fiber and its stiffness—by adding silk- or collagen-binding protein—will allow for the construction of materials with gradients in mechanical properties that can mimic tendons. The team also found that when they placed neural cells on the fibers, the cells aligned and grew axons along the direction of the fiber, which would be important in growing artificial nerves.
Jeffrey W. Ruberti, a professor of bioengineering at Northeastern University, says the “remarkable strength of the fibers” is similar to that of natural tissue, and could lead to clinical uses.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter