Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
An amino acid mystery
Lack of D-glutamate in mouse brains hints at undiscovered enzymes
by Louisa Dalton, special to C&EN
March 29, 2017
| A version of this story appeared in
Volume 95, Issue 14
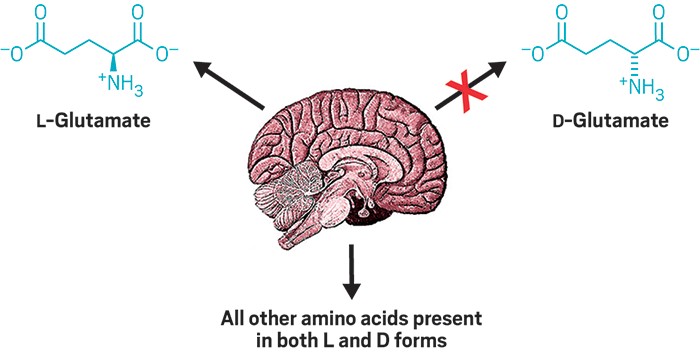
Right-handed amino acids—found in small doses in nature—are largely a mystery. In humans, most linger at low concentrations yet play unknown roles in the body. A new survey of right- and left-handed amino acids in mouse brains thickens the plot. The study’s findings imply that the brain tightly regulates the levels of right-handed amino acids and that undiscovered enzymes might flip lefties to righties (ACS Chem. Neurosci. 2017, DOI: 10.1021/acschemneuro.6b00398).
Ubiquitous left-handed L-amino acids serve as the building blocks of all proteins. The roles of their right-handed counterparts, D-amino acids, are still not fully understood. In 2000, scientists figured out that one of them, D-serine, is a neurotransmitter. But, to find the functions of others, scientists must conduct baseline surveys that determine normal levels of D- and L-amino acids in various parts of the body, says Daniel W. Armstrong of the University of Texas, Arlington.
Armstrong’s group collaborated with Adam L. Hartman’s laboratory at Johns Hopkins University to measure amounts of D- and L-amino acids in the cortices and hippocampi of mice. The collaborators separated blood from brain tissue and then purified the amino acids in the samples. They measured them by separating the D and L varieties with chiral high-performance liquid chromatography.
“Many curious things turned up,” Armstrong says. Levels of most of the 12 D-amino acids measured were 10 to 2,000 times as high in the brain as in the blood. The concentration of the neurotransmitter D-serine was among the highest, but D-aspartate and D-glutamine were even higher. Such quantities suggest many of the D-amino acids play an active role in the brain, Armstrong says.
Particularly striking was the conspicuous absence of D-glutamate anywhere in the brain or blood. L-glutamate is the most abundant amino acid in the brain—it is also a neurotransmitter—so Armstrong expected to see at least some D-glutamate. Yet his team couldn’t detect it at all with a detection threshold of 0.05 ng per mg of tissue.
The unexpected absence, Armstrong says, implies that the body keeps D-glutamate low for a physiological reason. It also implies that the brain has a mechanism for efficiently removing D-glutamate or keeping its levels very low.
Armstrong surmises that undiscovered enzymes may be at work. A known enzyme converts D- and L-glutamate to D- and L-glutamine, so perhaps a stereoselective enzyme takes L-glutamine back the other way but doesn’t take D-glutamine, making D-glutamine a sink for D-glutamate, he says. High brain levels of D-glutamine support that hypothesis, Armstrong says.
Herman Wolosker of Technion—Israel Institute of Technology notes that for some of the less abundant amino acids, relatively high fractions exist in the D form. Isoleucine, for instance, is 24% right-handed in the hippocampus. “It points to the possibility of additional enzymes in the brain that transform L- into D-amino acids,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter