Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Active catalytic site in methanol synthesis exposed
Atomic interface between zinc oxide and copper holds the key to converting CO2 and hydrogen to methanol
by Mitch Jacoby
April 10, 2017
| A version of this story appeared in
Volume 95, Issue 15

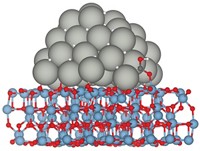
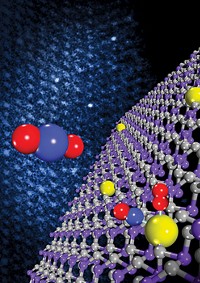
Industrial catalysts composed of copper and zinc oxide sitting on top of alumina can add hydrogen to carbon monoxide and CO2 to create methanol. But these catalysts have shortcomings, according to Ping Liu, a chemist at Brookhaven National Laboratory. The Cu-ZnO catalysts are not efficient or selective in producing methanol, and the reactions require high temperatures and high reactant pressures. What’s more, Liu said at the ACS meeting last week, chemical details of the active catalytic site remain elusive. Various researchers have argued that highly active Zn-Cu alloy species are the key catalytic players. In contrast, Liu’s new work suggests that the action occurs at the atomic interface between ZnO and Cu (Science 2017, DOI: 10.1126/science.aal3573). To reach that conclusion, Liu and coworkers prepared several types of Cu and ZnO reference catalysts, including one made of Zn nanoparticles deposited on copper and another with ZnO nanoparticles on copper. They analyzed and directly compared the CO2-to-methanol chemistry of all the catalysts using photoelectron spectroscopy and computational methods. The analyses showed that Cu-ZnO surface species are the most active form of the catalyst. They also showed that the Zn-Cu species don’t remain stable under reaction conditions. Instead, they react with oxygen and form Cu-ZnO.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter