Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Getting GPCRs into liposomes
Technique extracts the receptors from cells and puts them in proteoliposomes while maintaining their function
by Stu Borman
April 10, 2017
| A version of this story appeared in
Volume 95, Issue 15

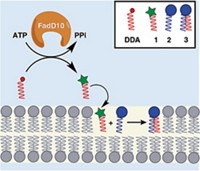
G protein-coupled receptors (GPCRs) are key drug targets and important signaling proteins embedded in cell membranes. To study the proteins’ specific signaling interactions more easily by eliminating the complexities of the cellular environment, researchers often use detergents to extract them from cell membranes and then reconstitute the receptors in synthetic membranes. The detergents can modify ligand binding interactions and other native properties of GPCRs, so researchers must use dialysis or adsorbents to remove detergents before studying the reconstituted proteins. A team led by Neal Devaraj and Roger Sunahara of the University of California, San Diego, developed a GPCR reconstitution technique that doesn’t require detergent cleanup (J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.6b12830). In the technique, called in situ lipid synthesis for protein reconstitution technology (INSYRT), the scientists use acyl maltose thioesters to extract GPCRs from cells, forming micelles containing the proteins. They then use native chemical ligation to combine acyl groups in the micelles with lysolipids, forming phospholipids that assemble spontaneously into spherical GPCR-containing proteoliposomes. The thioesters don’t survive the reaction and therefore don’t have to be removed. In a presentation at the ACS meeting, Devaraj showed that INSYRT could reconstitute the adenosine A2A receptor in proteoliposomes, where the receptor maintained its natural function.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter