Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Making biodegradable batteries out of silk
A silk-based battery could power implanted sensors or other medical devices
by Prachi Patel, special to C&EN
April 17, 2017
| A version of this story appeared in
Volume 95, Issue 16
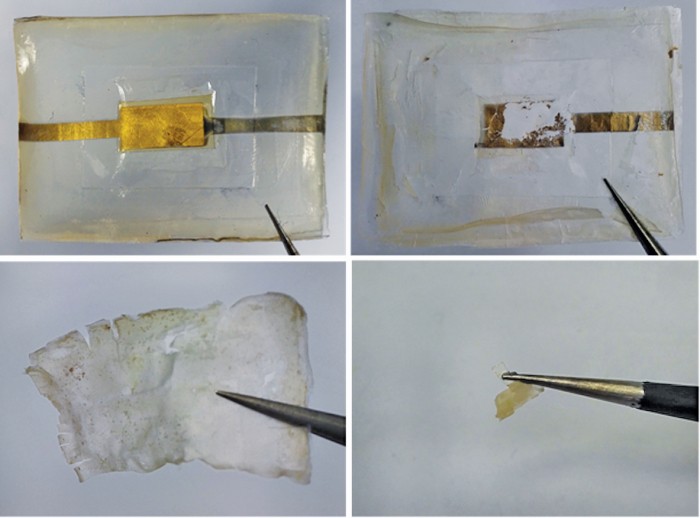
A flexible battery made of silk films could power temporary medical sensors and implants in the body and then harmlessly melt away once its work is done (ACS Energy Lett. 2017, DOI: 10.1021/acsenergylett.7b00012). Caiyun Wang and Gordon G. Wallace of the University of Wollongong and colleagues made thin silk films for the battery by dissolving the protein fibroin, derived from silkworm cocoons, in water. They spread the solution in a mold and peeled off ultrathin sheets of silk after the water evaporated. To make a solid electrolyte for the battery, the researchers infused a piece of the silk film with the ionic liquid choline nitrate, a molten salt that conducts ions. Depositing a magnesium alloy on another piece of silk formed an anode, and depositing gold on still another piece formed a cathode. The team assembled the battery by sandwiching the electrolyte film between the two electrode films. The postage-stamp-sized, 170-µm-thick device generated a voltage of 0.87 V, which would be enough to power an implantable medical sensor. When placed in a saline buffer solution, the device nearly completely decomposed after 45 days.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter