Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New way to make chiral arenes
Combined chemical and biocatalytic method installs group important in drug design
by Stu Borman
April 21, 2017
| A version of this story appeared in
Volume 95, Issue 17

A trifluoromethyl-substituted cyclopropane group can play a key role in a drug candidate. The group’s rigid cyclopropyl ring can increase the compound’s lipophilicity and metabolic stability while its fluorines can boost the candidate’s stability and ability to permeate membranes. But installing trifluoromethylcyclopropyl groups in a highly enantioselective manner is hard.
A team led by Rudi Fasan at the University of Rochester now reports a combined chemical and biocatalytic approach that could make it easier to add trifluoromethylcyclopropyl groups to compounds enantioselectively for drug design (J. Am. Chem. Soc. 2017, DOI: 10.1021/jacs.7b00768).
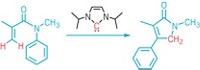
Erick M. Carreira of the Swiss Federal Institute of Technology (ETH), Zurich, and coworkers developed the only previous method for adding the fluorinated groups in a fairly stereoselective manner. In that technique, cobalt catalysts generate trans-trifluoromethylcyclopropyl derivatives of arene substrates with enantiomeric excesses between 84 and 94% (Angew. Chem. Int. Ed. 2011, DOI: 10.1002/anie.201004269).
Fasan notes that medicinal chemists prefer to use reactions with enantiomeric excesses greater than 98% to minimize the difficulties of purifying away unwanted enantiomers. Fasan’s new method provides enantiomeric excesses between 97 and 99.9% and has much shorter reaction times and 50 times the catalytic efficiency of the earlier method.
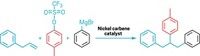
In the Fasan group’s technique, the researchers first convert trifluoroethylamine to a carbene donor reagent, diazotrifluoroethane. They then mix the reagent with bacterial cells expressing an engineered myoglobin, which catalyzes carbene addition to aryl olefin substrates, yielding trans-trifluoromethylcyclopropyl arenes. The scientists created multiple versions of the engineered myoglobins that can catalyze the synthesis of each of the two enantiomers for each addition product.
The chemical and biocatalytic reactions are not compatible. So the chemists carry out the two steps sequentially in two different pots to keep them separate.
Researchers have used myoglobin and other biocatalysts to transfer carbenes to substrates before, but those previous methods all used α-diazoacetate carbene donors. The new technique expands the range of biocatalytic carbene transformations to diazotrifluoroethane carbene donors.
“It is a great piece of work,” Carreira says. “It combines chemical and biocatalytic steps to make nontrivial, highly enantioselective cyclopropanation reactions possible. It is a win-win for chemistry, biology, and catalysis in general.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter