Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Weakness in perovskite crystals uncovered
Iodide salt coatings protect the promising solar-cell materials from attack by oxygen and light
by Elizabeth K. Wilson
May 22, 2017
| A version of this story appeared in
Volume 95, Issue 21

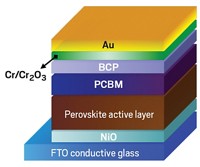
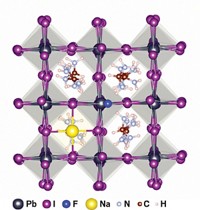
Methylammonium lead halide perovskites (CH3NH3PbI3) show great promise for solar-cell materials because they are more efficient at converting sunlight into electricity than current commercial materials are. Perovskite efficiencies can reach 22%, while those of commercial materials are about 15%. However, these perovskites suffer from a serious drawback: They degrade rapidly upon exposure to oxygen and light.
A group including M. Saiful Islam at the University of Bath and Saif A. Haque of Imperial College London now reports experimental and theoretical evidence for the mechanism behind this degradation (Nat. Commun. 2017, DOI: 10.1038/ncomms15218). Their findings have led them to a way to protect these promising materials.
The team previously determined that when light excites these crystals, it creates electrons, which can react with O2 to make reactive superoxide species. These superoxides then wreak havoc on the perovskite crystal structure, breaking it down to PbI2, methylamine, and water.
In the new work, the group uses computational simulations and experiments to show that O2 diffuses into iodide vacancies in perovskite crystal structures. These spaces were the most vulnerable and facilitated the superoxide degradation process.
With this mechanism in mind, the team tried to stabilize the material by coating it with a thin layer of iodide salts, which reduced the number of iodide vacancies. The protection was striking. Without the coating, the material began degrading within hours and became useless within days. However, crystals blanketed with the iodide salts remained stable for three weeks.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter