Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Janitorial system in red blood cells discovered
Enzyme helps trash most of the proteins inside cells that are transforming into red blood cells
by Sarah Everts
August 7, 2017
| A version of this story appeared in
Volume 95, Issue 32

Red blood cells are a stripped-down version of most other cells: The oxygen-carrying workhorses are packed with hemoglobin and little else. As immature red blood cells called reticulocytes transform into dedicated oxygen carriers, they trash their nucleus, organelles, and most proteins. Two groups of scientists are now reporting that this massive and selective clearance event occurs thanks to a protein degradation pathway involving an enzyme called UBE2O.
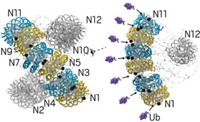
A team led by Daniel Finley and Mark D. Fleming at Harvard Medical School report that in the last stages of red blood cell differentiation, UBE2O remodels the proteome inside reticulocytes by tagging the small protein ubiquitin onto protein “trash” to signal for its breakdown, leaving the cell with approximately 98% α- and β-globin, the subunits that join to form hemoglobin (Science 2017, DOI: 10.1126/science.aan0218). Meanwhile, Ramanujan S. Hegde and his team at the Medical Research Council Laboratory of Molecular Biology in Cambridge, England, discovered that in reticulocytes, UBE2O also degrades rogue α-globin proteins that have not formed functional hemoglobin.
In other types of cells, Hegde’s team similarly found that UBE2O helps clear out orphan proteins that have also failed to join multiprotein complexes. The enzyme recognizes the proteins and tags them at many sites with individual ubiquitin molecules to signal for degradation.
This is unlike other ubiquitin-based degradation pathways, which tag proteins for destruction with long chains of ubiquitin molecules, explain Randolph Y. Hampton at the University of California, San Diego, and Catherine Dargemont at Paris Diderot University in an associated commentary (Science 2017, DOI: 10.1126/science.aao1896). “Further understanding of UBE2O and other quality-control pathways might open new therapeutic avenues” they add.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter