Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enzyme coordinates pericyclic reaction trifecta
LepI catalyzes three concerted rearrangements en route to the natural insecticide leporin C
by Bethany Halford
September 14, 2017
| A version of this story appeared in
Volume 95, Issue 37

Pericyclic reactions, in which electrons move in concert to rearrange a molecule’s structure, are standard tools for synthetic chemists. But examples of such transformations in nature are fairly rare. Chemists have now identified an enzyme that catalyzes three pericyclic reactions in the biochemical pathway that produces the fungal natural product leporin C.
“This really opens up the idea that nature is able to affect reactions of much broader generality than we ever knew before,” says Kendall N. Houk, a University of California, Los Angeles, chemistry professor who led the study along with UCLA’s Yi Tang and University of Shizuoka’s Kenji Watanabe.
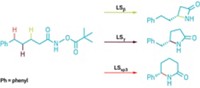
The enzyme, called LepI, begins by dehydrating a leporin precursor to generate a reactive intermediate. This intermediate, with the help of LepI, can either undergo a hetero-Diels-Alder reaction to produce the desired compound leporin C, or it can perform an intramolecular Diels-Alder reaction to produce a different intermediate that then is subject to a retro-Claisen rearrangement to produce leporin C (Nature 2017, DOI: 10.1038/nature23882).
When used by synthetic chemists, these pericyclic transformations often have to be performed at high temperatures, Tang notes. Also, he points out, it can be difficult to control the products’ regio- and stereochemistry. “As with any biocatalyst, this enzyme has potential to be engineered to catalyze such reactions under mild conditions with selective product formation,” Tang says. He also points out that leporins are deadly to insects, so this enzyme’s chemistry could lead to selective and potent insecticides either via synthesis of novel leporin analogs or large-scale bioengineered production of native leporins.
LepI is also noteworthy because the enzyme uses S-adenosyl-
“This places LepI on a short but growing list of methyltransferase homologs that catalyze unusual transformations of the carbon skeletons in their substrates rather than conventional methylation reactions,” comments Hung-wen (Ben) Liu, an expert in enzyme mechanisms at the University of Texas, Austin.
The chemists are currently using crystallographic techniques to try to find definitive experimental evidence for what SAM does in LepI.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter