Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
What’s the best way to show a new chemical reaction’s scope?
Organic chemists grapple with this question as standardized methods for substrate analysis come online
by Tien Nguyen
September 25, 2017
| A version of this story appeared in
Volume 95, Issue 38
When developing new reactions, organic chemists aim to construct molecular bonds in ways that have never been seen before. Once they’ve achieved this feat with a single set of reactants, though, their work isn’t over. To publish a novel synthetic method, researchers must demonstrate the reaction’s ability to work on different types of molecules, essentially proving that it can be useful in other settings and to other chemists.

Research groups satisfy this requirement by running their newly developed reaction on a series of compounds, called substrates, that contain functional groups such as alkyls, halogens, alcohols, or amines. Chemists record their reaction’s ability to transform various substrates, measured by reaction yield, in a so-called substrate table inside a manuscript prepped for submission to a journal.
But not all substrate tables in the chemical literature are created equal: Some contain results for under 10 substrates, while others have results for more than 100. Journals don’t have official requirements regarding the number or identity of substrates needed for publication, so these decisions, which are somewhat subjective, fall to the researchers introducing the method and manuscript reviewers.
The lack of consensus on substrate scope is an oft-discussed thorn in the side of the synthetic community. In the past couple of years, researchers have begun to propose more standardized approaches to help chemists evaluate new reactions and the substrates they’ll tolerate.
Last month, researchers at the University of Münster proposed a screening tool that could help chemists figure out in a matter of days how well their reactions might work with functional groups on various substrates. Led by Frank Glorius, the team assembled a set of 15 commercially available additives that, when introduced to a new reaction, could tell chemists how well a sampling of functional groups responds to a particular reaction (J. Org. Chem. 2017, DOI: 10.1021/acs.joc.7b01139).
Glorius readily admits that the additives approach, which builds on a “robustness” method reported earlier by his group (Nat. Chem. 2013, DOI: 10.1038/nchem.1669), simplistically tests functional groups rather than whole substrates. So it can’t tell chemists how the size, location, or electronic nature of the functional groups in the context of the entire molecule will affect a reaction, which is information typically provided by traditional substrate scope testing.
But the additive method can give researchers two independent pieces of information, he says. One, called functional group robustness, is how efficiently a reaction proceeds in the presence of a functional group, and the other, called functional group preservation, is how well a functional group survives the reaction conditions. When screening the additives—small acidic, basic, and nucleophilic compounds—on a reaction, chemists can use spectroscopic analysis to quantify the product yield and the amount of surviving additive to determine both of these parameters.
“Our method is absolutely complementary, so we don’t want to replace the substrate scope” testing, Glorius says. His group uses the additive screen only about half the time for papers, he says, and even then he leaves the decision up to his coauthors. “It’s suggested in more of a positive way, that if someone provides this screen, it’s a very honest way of showing what a reaction can do.”
A number of chemists in the community have seen the value in Glorius’s robustness method. Earlier this year chemists at Eli Lilly & Co., University College London, and the University of Pennsylvania led by Jeffery Richardson reported a method that takes Glorius’s original technique one step further (J. Org. Chem. 2017, DOI: 10.1021/acs.joc.7b00201). Using a high-throughput screening approach, in which reactions are run in 96-well plates, the team first tested the Buchwald-Hartwig amination, a classic C–N bond-forming reaction, against an array of functional group additives. They identified additives that worked poorly and then systematically optimized the reaction conditions in the presence of any “troublesome” additives, Richardson explains.
The researchers were able to raise the success of the coupling reaction with various functional groups by altering the reaction conditions. This strategy, they contend, can increase the diversity of compound libraries that can be tested against a certain biological target and thereby increase the opportunities for discovering new drug leads.
Richmond Sarpong’s group at the University of California, Berkeley, has also tried Glorius’s additives approach to help develop hydroamination and hydroetherification reactions for alkenes, forming new C–N and C–O bonds, respectively, en route to the total synthesis of alkaloids from Lycopodium plants (Chem.–Eur. J. 2015, DOI: 10.1002/chem.201406242).
To explore the scope of their method, the researchers would have needed six months or more to construct the substrates given their complexity and would have even needed to develop new chemistry to do so. “That’s why we decided to use the robustness screen,” he says, which took only a few days. “We got a little bit more than a qualitative sense of where our scope limitations lay and also overcame the investment needed to build the substrates.”
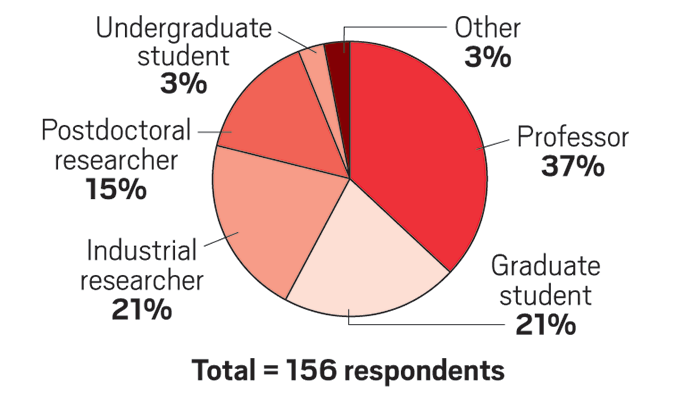
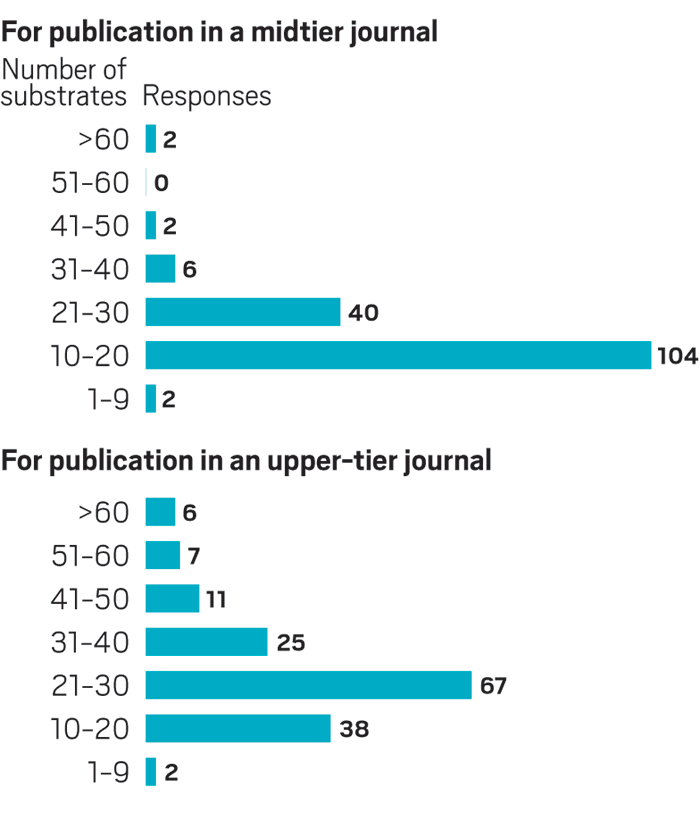
Survey results
C&EN recently polled organic chemists online about how they evaluate the number of substrates to test in new reactions. Here’s what they said:
Source: C&EN 2017 online poll
His group found the method useful, but Sarpong cautions that the results of the screen have to be “taken with a grain of salt.” He points out that reactions are always context dependent and in more complex natural product settings, multiple functional groups may nullify each other’s effects—for example, in cases in which both acidic and basic groups are present.
Medicinal chemists are interested in evaluating reactions against more complex substrates, too. Citing limitations in traditional substrate scope and additive approaches, chemists at Merck & Co. have introduced another strategy to compare the functional group tolerance of synthetic methods (Chem. Sci. 2016, DOI: 10.1039/C5SC04751J). The team constructed “chemistry informer libraries,” small sets of complex molecules that contain functional groups commonly encountered in drug development. Compounds in the library can require up to 15-step syntheses, making them fairly labor intensive to synthesize, says Merck scientist Spencer Dreher. The researchers screened well-established C–N and C–C coupling reactions against aryl boronate and aryl halide informer libraries and mapped the yields to reveal reactivity as well as patterns and trends for the reactions. The libraries are designed to be challenging “measuring sticks” for organic methods, Dreher says, adding that the team would eventually like to make these sets commercially available.
University of Illinois, Urbana-Champaign, chemist M. Christina White says using simple additives to evaluate a new reaction is a common practice for some labs but that Glorius’s method offers a way to standardize the results.
White, whose group has been developing novel reactions for more than 15 years, adds compounds with specific functional groups to new reactions as a way to prioritize substrate synthesis. “It’s just not something we ever thought to report on,” she says. Sometimes, a functional group will fail the additive screen but will end up working once incorporated into a substrate, and vice versa, she adds.
White says Glorius’s method is best used in concert with double-checking by making full substrates, but it could help a larger subset of chemists become aware of the general strategy.
Still, additive tools like Glorius’s won’t end the ardent debate over substrate scope among organic chemists.
In a recent online poll, C&EN asked chemists what they thought about the number of substrates needed to sufficiently demonstrate the scope of a new organic reaction. Of the more than 150 respondents, 41% said the chemistry community currently requires an appropriate amount of substrates, 25% said it requires too many, 20% said it requires too few, and 14% didn’t give a direct answer, citing problems with reaction scope beyond the number of substrates required.
In public forums and conference back rooms, some chemists complain that researchers “game the system” by testing substrates similar in identity and performance to increase the size of their substrate tables. A common example is testing one substrate with a methyl and one with an ethyl group with similar outcomes. This strategy might increase your table size and therefore get you into a better journal, chemists argue, but it doesn’t increase the value of the reaction or the data.
Over time, the number of substrates chemists publish along with new reactions has increased, although there’s no consensus on a sufficient range, particularly when considering the type of journal that might publish the work (see survey results).
Responding to C&EN’s poll, Eindhoven University of Technology’s Timothy Noël says it’s a good thing that standards are on the rise. However, he says, smaller research groups may suffer because they lack the resources needed to achieve the “monster” substrate tables now seen in elite journals. Noël’s group recently published a light-catalyzed decarboxylation method with 58 substrates (ACS Catal. 2017, DOI: 10.1021/acscatal.7b03019). The work was done by one graduate student and partly by a master’s student, which required a huge effort on their part, he says.
Uttam Tambar, a synthetic chemist at the University of Texas Southwestern Medical Center, says the strategy for evaluating substrate scope seems to have changed over the past decade or so, since he was a graduate student. “The way we were taught is every substrate in your substrates table should teach you something about the reaction,” says Tambar, whose lab has also used Glorius’s robustness screen as a time-saving measure (Nature 2017, DOI: 10.1038/nature22805). “Now it’s almost become a numbers game. People want to shock and awe you with the quantity of the substrates rather than the quality of substrates.”
Survey respondents echoed this desire for increased substrate quality—in the form of diversity and complexity—over quantity. It’s unclear, however, how the community would institute such a change.
For example, many chemists support the idea of being forthcoming and reporting substrates that fail in a reaction but hesitate to do so because it can lower the paper’s chances of publication, White says.
According to C&EN’s poll, 61% of respondents report that they publish substrates that didn’t work in a reaction, while 26% don’t publish them. The remaining respondents include failed substrates only in certain situations, oftentimes in the body or supplemental information of a paper rather than the main table.
“It would be great to see that culture change,” White says. She adds that showing the limitations of a reaction would encourage faster uptake of a researcher’s method by the community. Chemists won’t be left wondering whether certain functional groups work or whether they were just never explored.
Nessa Carson, an industrial organic chemist at Albany Molecular Research, responded to C&EN’s survey, saying that in the past she has contacted authors to ask if certain substrates worked in a reaction only to hear back that a substrate had worked, but poorly, and so it hadn’t been reported.
“Not only would I have loved to know (as I work on synthesis for medicinal chemistry), I’m more likely to have found their paper faster by doing a reaction search for the products!” she writes. “A low yield may not look nice in methodology, but in my job, commonly, there’s no other option.”
White suggests that changing the culture around disclosing substrate scope limitations could start with journal editors. They could make it clear to referees that it isn’t valid to reject a manuscript because of a few well-defined substrate classes reported as being low performing.
One survey respondent, graduate student Indrek Kalvet at RWTH Aachen University, suggested that journals should implement guidelines for reporting functional group tolerance. Glorius’s functional group screen could be officially recommended though not required, Kalvet writes.
“There’s no paradigm right now for exactly what to screen and why,” says Science deputy editor Jake Yeston. “It’s less a question of what do the journals want and more of what are the community’s goals in developing a new reaction,” he says. Generally, new reactions aim for one of two goals: Either demonstrate an entirely new idea or improve on or fill a gap in the reach of an existing method. A journal editor’s role, Yeston says, is to strike the fairest balance between the scope that’s needed in each case.
Advertisement
A representative from Organic Letters, Angela Hunter, tells C&EN that the journal doesn’t have specific guidelines for the number of examples for reaction scope analyses. But the journal does require authors to report isolated yields for reaction products to promote chemists’ ability to reproduce the results.
C&EN contacted journal editors from other publications but did not receive a response by press time.
Ultimately, reviewers often make decisions about whether a method has demonstrated sufficient scope based on intuition, Sarpong says. Regardless of whether a reaction has been evaluated with a robustness screen such as Glorius’s, Sarpong says, “people are always going to wonder about their particular substrate.” A standardized evaluation method isn’t necessarily a panacea, he adds, “but it’s certainly better than what people are doing now.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter