Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
China will accept foreign clinical trial data
Decision by country’s drug administrator should help foreign drug companies
by Jean-François Tremblay
October 11, 2017
| A version of this story appeared in
Volume 95, Issue 41

In a move likely to benefit Chinese citizens as well as foreign drug firms, the Chinese government has agreed to accept data from clinical trials conducted outside China for the approval of new drugs.
“Applicants can directly apply for drug listing registration after the completion of the international multicenter drug clinical trials,” the China Food & Drug Administration (CFDA) disclosed in a statement.
Until now, China only approved drugs that had been tested on people in China. And foreign firms could start testing in China only after they had demonstrated the safety of their drug with tests conducted overseas.
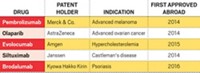
This meant that Chinese people gained access to innovative new drugs years after they had been approved overseas—if ever. In the past, many major drug companies did not bother to launch their drugs in China because of the cost and delay involved. By the time foreign-developed drugs gained approval, competing versions were often already available from Chinese firms.
CFDA has been reforming its drug registration process for several years. The agency earlier hired more reviewers to reduce its drug approval backlog. It also warned companies registering drugs in China to withdraw their applications—or face administrative sanctions—if they had doubts about the authenticity of their clinical trial data. The warning helped to reduce the number of applications that the agency had to review.
IMS Health, a market research firm, expects that China will account for 11% of global pharmaceutical spending by 2020. The country is already the world’s second-largest drug market after the U.S.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter