Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Mussel glues rely on cation-aromatic attraction
Protein adhesives that anchor the shellfish to rocks get their strength from cation-π interactions between amino acids, study suggests
by Michael Torrice
February 15, 2017
| A version of this story appeared in
Volume 95, Issue 8

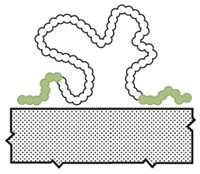
Mussels and other sea creatures that live along rocky shores often rely on special biological glues to fasten their soft tissues to rocks. These adhesives cure underwater and are strong enough to withstand the relentless forces of pounding waves. Scientists study the chemistry of these sticky proteins in hopes of producing new biomaterials, such as medical adhesives to attach muscle to bone during surgeries.
A team at the University of California, Santa Barbara, now reports that the stickiness of these mussel adhesives is, in part, due to attractive interactions between aromatic and positively charged amino acids. The researchers show that tweaking mussel-mimetic peptides with these interactions in mind can enhance adhesion (Nat. Chem. 2017, DOI: 10.1038/NCHEM.2720).
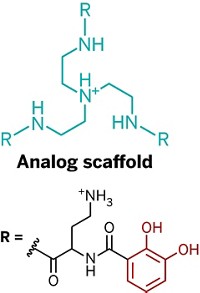
Previous studies of mussel proteins have found that two functional groups are critical to the adhesives’ function, says Matthew A. Gebbie, who is the first author on the new paper and is now a postdoc at Stanford University. The first is 3,4-dihydroxyphenylalanine (Dopa), which sports a catechol side chain that can cross-link with rock surfaces or other adhesive proteins through redox chemistry. The second is a cationic amino acid such as lysine. Not only do both functional groups sit close to each other in adhesive protein sequences, but synthetic versions of the glues also need both Dopa and lysine to be sticky.

Gebbie and his UCSB colleagues, including Wei Wei, J. Herbert Waite, and Jacob. N. Israelachvili, hypothesized that these two functional groups helped the adhesive proteins self-assemble and stick to each other through cation-π interactions. These noncovalent interactions involve electrostatic attraction between positively charged ions and electrons in the π orbitals of aromatic groups, such as Dopa’s catechol.
To test their hypothesis, the researchers studied synthetic peptides with sequences inspired by those found in mussel proteins. Each peptide contained lysine and one of three aromatic amino acids: Dopa, tyrosine, or phenylalanine. Based on solid-state nuclear magnetic resonance data, the team concluded that the positively charged ammonium groups of the lysines formed cation-π interactions with the aromatic side chains.
But the team was surprised when they measured the adhesive strengths of thin films of the peptides. “Given the prevalence of Dopa in natural mussel adhesive proteins, we suspected that the Dopa peptide would provide the strongest adhesion,” Gebbie says. In fact, the phenylalanine peptide exhibited an adhesion strength that was more than twice that of the Dopa peptide.
“The key insight from this work is the significant performance that can be obtained from cation-π interactions,” says Craig J. Hawker, who has studied catechol-based adhesive polymers at UCSB but was not involved in this work. “This adds another important design motif to the palette of building blocks for synthetic biomaterials and underwater adhesives.”
Gebbie also points out that some sea creatures, such as barnacles, don’t have catechol functional groups in their adhesive proteins. “We know there are other marine organisms that achieve strong underwater binding without Dopa,” he says. “We find it likely that cation-π interactions are playing a role there.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter