Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Pinning a bull’s-eye on cancer cells
Bioorthogonal technique adds azide groups to cancer cell surfaces for selective drug targeting
by Stu Borman
February 15, 2017
| A version of this story appeared in
Volume 95, Issue 8

A new small-molecule strategy could help target cancer drugs selectively to tumors with the help of click chemistry (Nat. Chem. Biol. 2017, DOI: 10.1038/nchembio.2297).
Many cancer drugs attack healthy tissue in addition to tumors, leading to harmful side effects. So scientists want to target therapeutics to cancer cells more selectively.
One such approach that has reached the clinic is antibody-drug conjugates. Antibodies recognize antigens, such as HER2 on some breast cancer cells, allowing antibody-attached drugs to kill those cancer cells selectively. But disease-specific antigens aren’t always available, and antibody-drug conjugates are costly and must be administered intravenously.
A group led by Jianjun Cheng of the University of Illinois, Urbana-Champaign (UIUC), Lichen Yin of Soochow University, and Xuesi Chen of Changchun Institute of Applied Chemistry has now come up with a strategy called active tissue targeting via anchored click chemistry (ATTACK) that they’ve tested in mice and that may have advantages over antibody-drug conjugates.
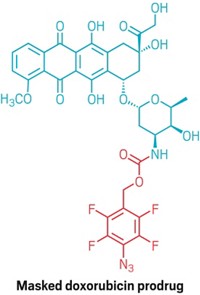
In the two-step ATTACK process, the researchers first give tumor-bearing mice an ether-protected sugar that carries an azide group. Cells can deprotect and then metabolize the sugar, which then gets attached to glycoproteins in the cell membrane. Because cancer cells proliferate quickly, they overexpress some enzymes, two of which catalyze deprotection of the azido sugar. This makes cancer cells more likely than normal cells to be tagged by the bioorthogonal azide groups.
In the second step, researchers give the mice an anticancer drug conjugated to dibenzocyclooctyne (DBCO). DBCO’s alkyne group undergoes a selective click-chemistry reaction with azides, thus recruiting the conjugated drug selectively to azide-decorated cancer cells.
In the mice, ATTACK improved drug-targeting selectivity 50% for treated tumors over untreated ones. And a DBCO-doxorubicin conjugate was significantly more effective and much less toxic than doxorubicin alone at treating colon cancer and two forms of breast cancer in mice—improvements the researchers plan to quantify in future work.
ATTACK has potential advantages over antibody-drug conjugates: It doesn’t require that a given cancer have a cell-surface antigen because the method creates its own targets; and ATTACK’s small-molecule agents could be orally available and less expensive to make.
The strategy “is very elegant” for achieving selective action because the ether is deprotected primarily in cancer cells, comments targeted drug delivery expert Liangfang Zhang of the University of California, San Diego. “It’s great work that will generate a lot of interest in the field.”
“The approach is clever in that it translates an intracellular molecular signature, cancer-related enzyme expression, to a cell-surface signature, azide groups that allow for targeting,” says bioorthogonal chemistry specialist Carolyn R. Bertozzi of Stanford University, adding that she is interested to see if the approach can be developed commercially.
Cheng and coworkers are founding a start-up called Iria Pharma through UIUC Research Park’s EnterpriseWorks Incubator to develop ATTACK clinically.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter