Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Engineering new cellular scaffolding for bacteria
Scaffold increases output of synthetic pathway by bringing enzymes close together
by Celia Henry Arnaud
December 18, 2017
Most cells have a network of protein filaments that help give the cells shape and organize their contents, among other important functions. These cytoskeletons vary in size and complexity: The scaffolds in bacteria are not as extensive as the ones found in cells from higher organisms.
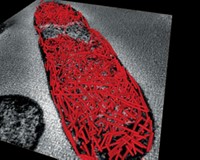
Researchers have now engineered bacteria with a more extensive scaffold that extends throughout the cells. The scientists used the scaffold to bring proteins closer together to increase the output of a synthetic enzyme cascade. Such an approach could aid the development of bacteria engineered for biomanufacturing of chemicals and materials.
The team, led by Martin J. Warren of the University of Kent and Derek N. Woolfson and Paul Verkade at the University of Bristol, engineered Escherichia coli to produce scaffolds made of a bacterial protein linked to designed coiled-coil peptides (Nat. Chem. Biol. 2017, DOI: 10.1038/nchembio.2535). The protein, PduA, naturally forms hexamers that assemble to form part of cellular structures called microcompartments in a different type of bacteria.
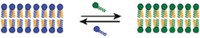
With the designed coiled-coil peptide attached to PduA, it instead produces short rodlike fibers from which the peptides extend. The fibers assemble into a scaffold that permeates the cell’s interior.
The researchers then can add a second coiled-coil peptide to the enzymes or other proteins that they want to attach to the scaffolding. This second peptide contains positively charged, acidic amino acids that can interact with the negatively charged, basic amino acids on the peptide attached to PduA.
The researchers used the scaffold and tagging strategy to bring together the enzymes pyruvate decarboxylase and alcohol dehydrogenase, which are involved in the production of ethanol. They found that scaffold-containing bacteria produced about half as much of the enzymes but twice as much ethanol as bacteria without the scaffold.
“We can literally pin tens of thousands of enzymes to these cytoscaffolds,” Woolfson says. “This means that we can engineer extremely high densities of active enzymes in the cell.”
Oliver Yu, chief science officer at Conagen, a company that is engineering bacteria for biomanufacturing, thinks that the new scaffolding approach will be versatile. “There are no clear limitations on how many enzymes we can bring together,” Yu says. “The approaches for building this scaffold are simple and straightforward.”
The researchers are extending the approach to other multienzyme systems. In addition, they are targeting the scaffolds to specific parts of the cell and introducing other designed peptides and assemblies into cells.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter