Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Feast your ears on the first episode of our new podcast, Stereo Chemistry
Hear from some of the leaders in the field of metal-organic frameworks as we learn why MOFs are having a moment
by Matt Davenport
February 28, 2018
| A version of this story appeared in
Volume 96, Issue 10
Metal-organic frameworks are so hot right now, and we wanted to know why. In our very first full-episode of Stereo Chemistry, we talk to Omar Yaghi, who recently earned a share of the Wolf Prize in Chemistry for his MOF work; Omar Farha, who helped build a company around MOFs; and other chemists not named Omar to better understand these materials.
Subscribe to Stereo Chemistry now on iTunes, Google Play, or TuneIn.
The following is a transcript of this podcast.
Matt Hartings: It’s gonna have to warm up. If anything, it’ll say warming up.
Matt Davenport (voice over): Hello and welcome to the very first episode of C&EN’s new podcast, Stereo Chemistry. I’m Matt Davenport and the person you just heard is Matt Hartings, a chemist at American University.
Matt Hartings: Not responding? Uhhhh.
Matt Davenport (voice over): But we’re actually at the National Institute of Standards & Technology, where Matt was on sabbatical this past fall. And you might already recognize the sound you’re hearing. It’s the sound of research. It’s the sound of something not working. Until…
Matt Hartings: Oh there we are. It’s working on it.
Matt Davenport (in lab): Gasp. Oh, it’s movin’.
Matt Davenport (voice over): We were standing over his 3-D printer, waiting for it to start up. And it’s just a regular 3-D printer. You know, the kind that heats up plastic and then squeezes the polymer through a nozzle to build up one-of-a-kind plastic things. And I don’t mean to downplay how cool regular 3-D printers are. People have created all sorts of neat and important things with regular 3-D printers—things like models of molecules and proteins, things like cheap, on-demand replacement parts for appliances, things like medical implants designed for specific patients.
But at the end of the day, those things are still just plastic. And that’s why we were with Matt Hartings in a lab at NIST. Although his printer is pretty standard, the materials he prints are not. And we’re here to find out why he’s printing plastics laced with metal-organic frameworks, or MOFs.
Matt Hartings: So, I have always been interested in 3-D printing. There are all these stories of people who have 3-D printed prosthetics and other amazing 3-D printed things. But when I look at 3-D printing, I do it from my position as a chemist and I see these 3-D printed things and I think, “Well, chemically they’re pretty boring, right. You print them and they are chemically inert they don’t do anything else.” And so I thought that, y u know, as chemists, we’re missing out on some of the fun in 3-D printing. And so I wanted to find a way to 3-D print things that did chemistry after they were 3-D printed.
Matt Davenport: Enter metal-organic frameworks. There are MOFs out there that do all sorts of chemistry. There are some that are great for catalysis. Others can do gas sensing, storage, or separation. MOFs are a versatile and exciting platform for chemists.
And that’s what we’re going to look at today in the first full episode of our podcast. MOFs are having a moment right now and we wanted to really understand why that is. And, honestly, I think the best answer that I have comes down to what might sounds like a pretty boring word: Stability. But I’m here to tell you it’s not boring. There is so much history and potential and just chemistry packed into that one little word. So we’re going to spend the next half hour or so unpacking that excitement with some of the people making the most of MOFs.
Which brings us back to that NIST lab with Matt Hartings. He’s working with a MOF that’s promising for storing hydrogen, an element that could provide us with a clean, sustainable fuel source. And Matt’s team worked that MOF into a polymer composite, which the researchers can then heat up to like 200 degrees Celsius and 3-D print into arbitrary shapes.
For example, they printed these little perforated plastic towers that have oodles of surface area for gas storage. They also printed what look like Lego bricks and what scientist wouldn’t be thrilled to get a box of chemically functionalized Legos for their birthday? But the idea is you’ve now got all sorts of flexibility to arrange MOFs how you want them.
And MOFs come with their own inherent flexibility. And that will make a little more sense once I tell you what a MOF is. While reporting this story, I heard lots of amazing descriptions of MOFs—and this is not one of those. But it will work for now and I promise you’ll get a more complete picture of MOFs as the podcast goes on.
So here goes. Imagine a sponge designed by the most anal-retentive engineer in the universe. This sponge would not only optimize the amount of empty space it has, but it would do that by using this stable, ridiculously ordered framework around those empty pockets. And now, chemists have developed synthesis techniques that bring inorganic metal clusters together with organic linker molecules such that nature does the anal-retentive engineering for them.
Furthermore, chemists can tweak MOFs to get specific properties for specific applications. And they can do this by changing the MOF’s organic chemistry, or its inorganic chemistry, or both. MOFs are a designer material—a designer material that’s now combined with the flexibility of 3-D printing.
Matt Hartings: I can change the polymer that I’m using. I can change the MOF that I’m using and I could also change the geometry that I print in. And I can change all of those things to go after different applications. If that application space is gas filtration I’m going to print a different object than I would if I were trying to do a gas storage and release. And so I can start to play around with structural geometry. And printed geometry in a way that maybe I haven’t been able to before as a chemist. I can start to see how these different geometries that I print in will facilitate the types of chemistry that I want to do.
Matt Davenport: There are just so many choices, so many options. I wonder if we know anyone who can describe what that feels like…
Omar Yaghi: It’s almost like a kid in a candy shop.
Matt Davenport: That’s Omar Yaghi, who is a professor at UC Berkeley and one of the founders of modern MOF chemistry. He actually just won a share of the Wolf Prize in Chemistry for his MOF work. So who better than him to set the stage for us on how MOFs got to where they are today.
Omar Yaghi: I sometimes say MOFs are the largest class of materials ever made by humans. And that’s because you have exploited the periodic table of the elements, the transition metals and the organics. And we figured out a way of linking those two together.
You know, when I got interested in this area, I was really interested in the beauty of these frameworks. I knew deep down that if you could stitch organics and inorganics together and make very stable frameworks then you could apply all the beautiful molecular chemistry that has been developed over the last hundred years on materials. And therefore transforming not just the way you make materials but the way you use materials.
So, what are the limitations on this? The limitation in my opinion is really our own thinking. For the first time in my career as a chemist you can imagine a structure and go to the lab and make it. And when I was a student if you imagine such a structure went to the lab to try to make it you got amorphous stuff that is uncharacterizable and now we have figured out a way of making these as beautiful crystalline material that can be characterized fully. We know where all the atoms are and we know how they’re connected and that’s why the progress has been rapid.
Matt Davenport: Hearing this, MOFs blurred the distinction that I saw between molecules and materials. You know, chemists control which atoms or functional groups go where in a molecule to dictate its function. And that level of atomic control is necessary to design molecules, but not so much for bulk materials. For materials, researchers develop processing techniques that keep impurities or imperfections low enough to deliver the properties they want, say mechanical strength or electrical conductivity. Or, once they have an imperfect material, they can work on it with post-processing steps to improve the material’s functionality.
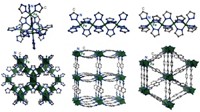
Now there are MOFs, which are ordered materials that you can basically synthesize from the bottom up. And fairly easily, too. And let’s actually take a closer look at one of the most famous MOFs, called MOF-5, to give you a better idea of what a MOF is.
MOF-5 has a simple cubic crystal structure, or primitive cubic if you want to be technical and a little judgy about it. The crystal is a large cube made from smaller, identical cubes with an atom at each vertex. The bonds between the atoms make the edges. Fun fact: Did you know that polonium is the only pure element with this crystal structure? That was news to me...soooo shout out to polonium.
But I have a hard time imagining things I can’t see, so for this exercise, imagine a cubic crystal made of marshmallows and toothpicks. Just a giant cube made out of smaller cubes with a marshmallow at each vertex, connected by toothpicks. The marshmallows, then, put the metal in MOF. And the toothpicks are the organic part.
In MOF language, the marshmallows would be the anchors or secondary building units, the toothpicks are the organic struts. In MOF-5, the anchors are octahedral zinc oxide clusters. And the struts are 1,4-benzodicarboxylic acid, which is basically a benzene ring with two stubby arms on either side to grab onto zinc oxide anchors.
Now, MOF-5 is a single, simple example of MOFs. There are more than 60,000 MOF structures according to one count from the Cambridge Crystallographic Data Centre. And these MOFs can get way more complicated than MOF-5, like diabolically so. But even as a simple example, MOF-5 illustrates something shared by all MOFs. They’re mostly empty space. There’s not a lot of meat on those metal-organic bones. Yet those bones are strong and that makes today’s MOFs pretty stable. That wasn’t always the case.
Omar Yaghi: Okay, so metal organic extended structures have been known for a long time. In fact, if you look in the literature, back in the late 50s, the Japanese were working already on copper one linked with an organic linker that has nitriles at the end of it to bind to the copper. And they made these extended frameworks. And many others following their footsteps have come up with similar structures: nitriles or bipyridine, linked by copper or transition metal.
When I came along, I started doing exactly the same thing. I took copper one and bipyridine struts and we made these compounds. That was the early 90s, almost mid 90s. And you made these beautiful structures and they were you know they were like sculptures. But very quickly we realized that they’re not very useful. Because as we tried to take advantage of the openness within these structures, the structures collapsed.
Matt Davenport: And good luck storing hydrogen in that, am I right? At any rate, it was obvious that these early frameworks were unstable, but Omar came to realize why. He tells us the bonds were to blame. The organic linkers they were first using, the nitriles and bipyridines, are neutral Lewis bases. This means that their interactions with transition metals are weak. So Omar and his colleagues wondered what if they moved to a charged linker, something like a carboxylate?
Advertisement
Omar Yaghi: So two things that the carboxylates gave us. One is that they gave us now instead of a transition metal-neutral linker, now you have a transition metal with a charged linker that’s a much stronger bond then the nitriles and the bipyridines. But also the fact that these carboxylates came together in aggregated metal ions made metal oxide clusters as anchors. Instead of a single metal ion, now you have clusters and these clusters are rigid and they are made entirely of strong bonds. So then we made the first what we called metal-organic framework.
Matt Davenport: In 1995, Omar and his team showed for the first time that they could move benzene molecules in and out of a MOF without the MOF falling apart. Then they delivered another first in 1998 with a MOF they called MOF-2. Using MOF-2, they measured what’s called a gas adsorption isotherm. It’s a measure of how much gas the MOF absorbs at a constant temperature under varying pressure. These experiments let Omar and his team figure out how much open space was available per gram of their MOF.
Omar Yaghi: This experiment was critical because it allows you to get porosity parameters like surface area. And for the first time we were able to compare MOFs to the more established porous materials. But the world didn’t take notice of this development until we made MOF-5 in 1999 because MOF-5 that broke all records of porosity. We reported twenty nine hundred meters squared per gram. And that was a lot higher than people were able to obtain for zeolites, with mesoporous materials, and porous carbon.
Matt Davenport (interview): So MOF-2 was important. MOF-5 set records. Are there any interesting stories about MOF-3 or MOF-4?
Omar Yaghi: Well, MOF-3 was the first MOF where we pointed out the importance of open metal sites.
Matt Davenport: If you think of a MOF’s transition metal sites as inns or hotels for coordination chemistry, open metal sites have some vacancy or capacity left. They haven’t filled up with up with organic ligands.
Omar Yaghi: Open metal sites now are very important for separation of organic molecules and in enhancing the hydrogen uptake, methane uptake, carbon dioxide capture. So there’s a lot of work that’s being done at utilizing these open metal sites for those applications but also for catalysis.
Matt Davenport: And I just realized he kind of dodged my question about MOF-4. Man.
Still, within their first five MOFs, Omar and his colleagues basically set the tone for the field. They put together these stable, versatile structures that could be chemically active and had massive amounts of storage space. And things took off from there.
Omar Yaghi: You can get surface areas up to 7,000 meters squared per gram. That is in one gram of material you have the footage of an entire football field. That is the space onto which gases can bind within the material.
Matt Davenport (interview): When you say in one gram, there’s the surface area of a football field, is that American football or the international football?
Omar Yaghi: It’s actually more it’s actually more than one football field. So either one would work. It’s more than a soccer field or the American football field. In one of the MOFs, we call it the Queen of MOFs, MOF-177, had of porosity of about 5,000 meters squared per gram. And we showed that in fact in a tank filled with MOF-177, you could store double the amount of hydrogen that you would store in a tank that does not have the MOF. And so even though the MOF was occupying volume, the MOF pores were acting to compact the gas molecules within the pores. And so this was, this was amazing.
Matt Davenport: Omar and his team have already made more than 1,000 different MOFs. And I don’t want to sound like I’m not impressed by that, but it sort of makes sense after learning how versatile MOFs are as a platform. You can change the metal anchors and the organic linkers, and still do post-processing to get at the functionality you want for a specific application. What does blow my mind, though, is that Omar wasn’t really thinking about applications when he first got into MOF chemistry.
Omar Yaghi: One CEO from Nalco Chemical Company came down to see what’s going on about, you know, MOFs were just beginning to be noticed by the community and he wanted to see what is all the fuss about. So he came down I was still assistant professor and I was showing him all these beautiful structures and describing their intricate connectivities and their porosity. And then he at the end of my little lecture that I was giving him he said, “Well, what are they good for Omar?” I thought, “Here I am establishing a fundamental area of chemistry and this is an intellectually stimulating world of beautiful new structures and he’s asking me how I could apply these to society.” And I thought, I’m supposed to be a scholar, an intellectual that just produces knowledge and let society deal with it.
Matt Davenport: But the CEO then said something that changed everything for Omar. He flat out said Omar would never be a great professor until he brought MOFs to benefit society. And you can definitely see the impact that conversation had on Omar in the types of applications his lab pursues. The group has built MOFs to store methane and hydrogen, to capture carbon dioxide, and even to harvest water. But other engineered nanomaterials—things like graphene and nanotubes—are also being studied for these applications. What sets MOFs apart?
We’ll get you an answer in just a second, but first, I just wanted to thank you again for listening. We are so excited about this podcast and about the fact that you are listening to it. We’re also really excited about the stories we’re going to tell here in the future, but we’re going to be a monthly podcast after our first three episodes. So how can you stay up-to-date with the chemistry news you need to know?
Dorea Reeser: They could subscribe to our newsletter.
Matt Davenport: Well hello there, C&EN audience engagement editor Dorea Reeser.
Dorea Reeser: Hey Matt.
Matt Davenport: Can you tell us a little bit more about the newsletter and how to get it?
Dorea Reeser: Well you can get our newsletter by opening your web browser and typing cenm.ag/cenewsletter. What can you get by signing up for our newsletter? Well, not only will you get the latest news, but sometimes we actually set up some special features, such as what’s going in policy or in the workplace for chemistry. You can also expect to see some really cool extra content, such as Periodic Graphics, which are in collaboration with Compound Interest. Or Chemistry in Pictures, which features submissions from you guys.
Matt Davenport: Great. So can you remind us where we can sign up for the newsletter?
Dorea Reeser: Absolutely. You can sign up for our newsletter at cenm.ag/cenewsletter.
Matt Davenport: Is there anything else we need to know?
Dorea Reeser: Hmmm. Not about the newsletter, but I am dying to know how MOFs are different from things like graphene and nanotubes.
Katherine Mirica: With metal-organic frameworks, there’s this unique possibility and capability to synthesize them bottom up, using chemical methods and very uniquely structured molecular building blocks. So from a synthetic chemistry perspective there’s a lot of modularity and creativity that can go into the design of these materials that gives them new emergent function, compared to carbon nanotubes or other materials that have to be made and then functionalized.
Matt Davenport: You’re probably wondering who that was.
Katherine Mirica: I’m Katherine Mirica. I’m an assistant professor in the chemistry department at Dartmouth College.
Matt Davenport: Like Matt Hartings, whom we spoke with earlier, Katherine is also working to integrate MOFs with other materials. And moving to these sorts of composites feels like the next step in MOF evolution. Twenty years ago, the question was how do you make stable MOFs. Now chemists like Katherine are asking how do you work with them, how do you process them, how can you weave them into tapestry of materials we rely on every day? For Katherine’s team, one of the ways is using electrically conductive MOFs with fabrics to make gas sensors and other devices.
Katherine Mirica: Using the properties of the metal-organic framework, what they’re already known for in terms of porosity and filtration and capture, but using that in conjunction with electronic transduction, I think, offers a lot of possibilities. Metal-organic frameworks really offer very unique advantages in separations and storage of gases, as well as filtration and sensing. And I think the realization of that full potential really requires efficient solid state configurations of these materials and often composites with other materials.
Advertisement
Matt Davenport: Katherine then told me how she thinks about the MOF-fabric composite as having this sort of hierarchical architecture, which is really hard for me to say out loud, but really fun to think about. MOFs have pores at the nanoscale and fabrics are porous at the macroscale. Bringing those two levels of porosity together gave some interesting results.
Katherine Mirica: Fabrics we think offer a very unique capability of porosity that can be integrated and harnessed in conjunction with the porosity of the metal-organic framework. We actually see some really nice performance in terms of sensing, which we attribute to the dual porosity of the fabric and then being able to template the metal-organic framework on top of that.
Matt Davenport: And I couldn’t help myself. Any time I talk to somebody about electronic materials and fabrics, I compulsively ask if they’re making new wearable technology.
Katherine Mirica: I think there are no reasons not to do that. We’re definitely going in that direction and I think this fabric-based platform is the first demonstration of the ability to integrate conductive MOFs into fabrics. MOFs have already been put onto fabrics there some beautiful precedent showing that but they have not been conductive MOFs and so being able to conformally coat fabrics and generate electronic textiles with metal-organic frameworks I think offers definitely some new possibilities for wearables.
Matt Davenport: As cool as I think it would be to wear MOFs around, the part of my conversation with Katherine that stuck with me the most was hearing her describe what she called emergent behaviors. In her case, that was seeing MOFs become better sensors when integrated with her fabrics. And many other researchers are working to explore these behaviors with other composites, for instance, by combining MOFs with glasses or polymers.
And again, the idea is that a MOF composite delivers more performance than either the MOF or the matrix material alone. And that matrix material—the fabric, the glass, the polymer—could actually make up for some of MOFs’ shortcomings.
I know. That’s an about face after pulling the MOF bandwagon for this entire episode. But MOFs aren’t perfect. For instance, when you synthesize MOFs, you typically end up with powder and that’s not great if you’re trying to precisely engineer some sort of fancy device that uses MOFs. Here’s Matt Hartings again.
Matt Hartings: It’s difficult to just take a powder and throw it around and have it land on a device exactly the way you want to and have it stay in place. You want something where you can control the geometry, control the amount. You need to be able to have process control.
Matt Davenport: And although MOFs are more stable than their predecessors, remember, their predecessors were bad. So they’re more stable than not stable, but that doesn’t always mean stable.
Matt Hartings: You know MOFs, while they are a really interesting class of materials, many of them are not stable in extreme conditions. Probably the prototypical MOF, which is MOF-5, will start to degrade at 60% humidity. And so that’s not super extreme.
Matt Davenport: So Matt and his group did some experiments with 3-D printing MOF-5, they wrote up a paper, and they submitted it. And then they got reviews.
Matt Hartings: And we hadn’t, I guess we hadn’t figured out that the humidity in our building at American University, you know, it’s always at least 60%. Sixty percent is the low threshold for where our humidity is. D.C. is a swamp in general and the epicenter of that swamp is American University. It’s always super humid and our MOF was degrading before we put it through the 3-D printing process. So I think that was one of the biggest lessons that we needed to learn is how do we deal with humidity. The pieces that we have now, we can take them and plunge them into water for a week and the MOFs inside of the polymers don’t fall apart. So that’s a big step forward in our eyes.
Matt Davenport: So just to reiterate: Two of the reasons today’s MOFs are remarkable are that they’re porous and that they’re stable. Now, researchers are combining them with other materials that can add more porosity and more stability. And they can do this with materials we can already produce and process en masse. So the potential isn’t just to create composites that are exciting, but ones that are also accessible.
And I don’t want to get ahead of myself. It’s not like you can run out now and buy MOF Legos to build your own methane storage tank. It will be awhile before that happens, if it ever happens. But this sort of material synergy helps explain why MOFs are having a moment. There’s a lot of promise, both in terms of applications and the science to be learned.
Matt Hartings: I foresee us continuing to develop new ways to incorporate MOFs into larger materials, no matter what that material is, whether it’s a polymer—things that I’m working with—or a it’s fabric or whether it’s something else, right? How do we get those MOFs into a usable space? I think there’s a lot of work to be done there and so there’s really interesting chemistry that’ll make that happen.
Matt Davenport: So that’s the type of forward-looking quote that would be perfect to end a podcast with. But MOFs are having a moment right now. What kind of host would I be if I didn’t tell you MOFs are being commercialized right now. Well, actually, I’m not the best person to tell you that.
Omar Farha: Hi Matt. This is Omar Farha from Northwestern. How are you?
Matt Davenport: In addition to being a MOF researcher at Northwestern University, Omar Farha is the cofounder and chief science officer of NuMat Technologies, which was one of C&EN’s 10 Start-ups to Watch in 2016. Last summer, NuMat partnered with Versum Materials to create gas cylinders with MOFs to store toxic gases used in the semiconductor industry, namely arsine, phosphine, and boron trifluoride. Using these MOFfed up cylinders, semiconductor makers can store these hazardous gas below atmospheric pressures. That’s safer than traditional gas cylinders that store gases above atmospheric pressure.
Now, Omar concedes that developing toxic gas storage solutions represents a smaller market, Omar concedes. But it’s still a commercial market. So this answers some of the questions people have had about being able to make MOFs at a commercial scale. And, as has been the case with MOFs from the beginning, once you answer those fundamental questions, you build from there.
Omar Farha: We already showed that MOFs can be scaled. You could go and make it at a price that is competitive with others absorbents and you can make it at a scale that you can handle a smaller market. Now, the next thing is go to the next markets which are larger, different storage applications or different separation applications, that might require knowing how to make the MOF even cheaper or forming the MOF with a specific formation.
You know, MOFs, when people started, you have to start somewhere. And you have to show the world that you could build them, you could build them by design. And show porosity and show that you could make it and you could measure uptake and release of certain gases. And that was the main thing, let’s say 20 years ago.
Matt Davenport: Fast forward to now.
Omar Farha: We are really in the right time for the MOF that you have materials that they are thermally stable. They are water stable. And some families are base stable. Other families are acid stable. And these families of materials weren’t there 20 years ago because the goal wasn’t let’s worry about that type of stability. But now because we have these materials, that allows to go after the hard applications. Right now, the MOF field has those materials to go after those challenges, which to me is very exciting.
Matt Davenport: I asked Omar to check out the papers from Matt and Katherine’s groups, showing how they worked different types of MOFs into different materials with different processing techniques. And he was really excited. But that got me thinking, you know, is that Omar the Northwestern Researcher talking? Does start-up cofounder Omar worry about these types of MOF materials making it out of the lab to potentially compete with NuMat?
Omar Farha: To be honest with you, at NuMat we don’t think of as other start-ups in the MOF field as competitors. We really do want more startups to show up. And we really do want more startups to succeed because that’s good for everybody. That’s good for the field. That’s good for the investors. It’s a win-win for everybody. MOFs are a big platform. Different startups going after different applications, they’re not getting in each other’s way. Is that going to be the case forever? Maybe not. But for now, I think the more MOF start-ups succeed, the better for everybody. And I think start-ups in general should be each other’s cheerleaders and not think about each other as competitors. At least that’s the way we see things as NuMat.
Advertisement
Matt Davenport: And hearing this took me right back to my interview with Omar Yaghi at UC Berkeley. MOF research had also taught him something about the importance of support in science.
Omar Yaghi: So along each step there was criticism and detractors, but the nice thing about the life of a scientist is that there’s always some people out there that appreciate what you do and they’re not afraid to stand up and say, “This is interesting work. This is worthwhile work.” So I learned, now that I am a senior professor, senior researcher, that whenever I see something nice that a young, emerging scholar or assistant professor or somebody starting out their career, when they are doing something truly groundbreaking is to say, “This is nice work. Keep going.” And and I was fortunate to have that. There were people out there just like I had that CEO come into my office and propelled me to take these materials to applications. So this is this would be my advice for any anybody doing research. It’s research in itself is a very difficult endeavor because you fail a lot. And the reason you keep going is because of the potential success that you might get along the way. But during this process you will have a lot of detractors, but don’t forget that you will also have supporters. And that’s what counts. The detractors you have to listen to what they’re saying because you have to address any weakness in your work that you might have. But there’s always people out there who do make constructive contribution to your research by simply talking to you about the good and the bad in what you’re doing.
Matt Davenport: So that’s it. That’s the real concluding quote of the first episode of Stereo Chemistry. We’d love to know what you thought of it. This is obviously early days for us and we’re going to experiment with telling different stories, different ways, and we’d love to know, like Omar Yaghi said, the good and the bad in what we’re doing. You can tweet at me @MrMattDavenport and my cohost Kerri Jansen, whom you’ll hear from next week, is @absoluteKerri. Do not miss her episode. She’s following up with the people behind C&EN’s cover story on sexual harassment to learn what’s happened since the story came out.
So subscribe to Stereo Chemistry now on iTunes, Google Play, or TuneIn and leave us some feedback while you’re at it.
The music you heard first in the episode—and what you’re hearing now—is “What Have You Done” by Lee Rosevere. And the music you heard during our chat with Dorea Reeser about the newsletter was “The Confrontation” by Podington Bear. We’ve got links to the songs and their licensing information in the episode description.
For C&EN, I’m Matt Davenport. Thanks for listening.
Fin.
Here are links to some of the publications we mention in the podcast, as well as some other resources that we found useful, interesting, or just plain fun during our reporting of this episode.
Toward 3D printed hydrogen storage materials made with ABS-MOF composites | Poly. Adv. Technol.
Design and synthesis of an exceptionally stable and highly porous metal-organic framework | Nature .
Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases | J. Am. Chem. Soc.
Metal–Organic Frameworks from Edible Natural Products | Angew. Chem., Int. Ed.
Reticular Chemistry Naming and Numbering Database | UC Berkeley
“What Have You Done” by Lee Rosevere is licensed under CC BY 4.0.
“The Confrontation” by Podington Bear is licensed under CC BY-NC 3.0.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter