Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Chemists break C–C bond record
New hydrocarbon compound contains a 1.806-Å-long bond that stretches beyond theoretical limit, researchers claim
by Tien Nguyen
March 8, 2018
| A version of this story appeared in
Volume 96, Issue 11
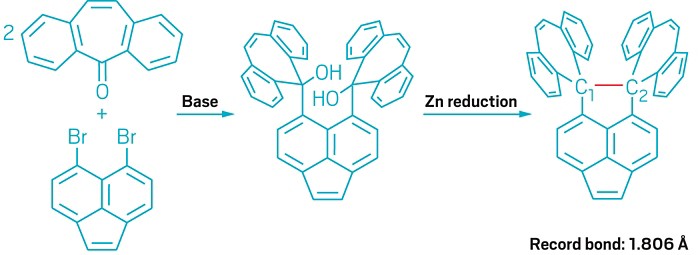
The single carbon-carbon bond is among the most familiar covalent connections in organic compounds. But a new study suggests that chemists have yet to fully explore the limits of this basic bond.
Scientists have previously found that these ubiquitous bonds, which typically measure about 1.54 Å in length, can be elongated through the use of bulky substituents to endow molecules with special properties. Now, researchers led by Takanori Suzuki and Yusuke Ishigaki at Hokkaido University have synthesized a molecule they claim has the longest reported C–C bond among neutral hydrocarbons, measuring 1.806 Å in length (Chem 2018, DOI: 10.1016/j.chempr.2018.01.011).
The team set out to break the record by designing a stable dihydropyracylene compound with a highly strained core and two spirocyclic units that are forced to face each other, helping stretch out the central carbon-carbon bond. The chemists confirmed the presence of the C–C bond by observing its stretching vibration through Raman spectroscopy, and they measured the molecule’s record bond length using X-ray crystallography.
The bond breaks the theoretical limit of 1.803 Å, previously calculated for caged dimer compounds. This limit is set at the point at which the bond has a dissociation energy of zero and would thus dissociate. Chemists calculated the limit by assuming a linear relationship between bond length and bond dissociation energy. The Hokkaido researchers suggest that molecules like theirs with extralong bonds may deviate from this linear relationship.
Structures with extremely long or short bonds help us refine our understanding of chemical bonding, Peter R. Schreiner of Justus Liebig University Giessen says. He thinks work like this, which pushes the limits of bonding, is worth careful consideration. “It also urges us to keep asking the question, When is a bond a bond?”
CORRECTION: This story was updated on April 4, 2018, to correct the specifics of the record broken in this work. The researchers claim their molecule has the longest C–C bond among neutral hydrocarbons. The bond is not an alkane bond. Also other teams have reported longer C–C bonds in charged molecules and in nonhydrocarbons.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter