Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Organometallic flow reaction reaches industrial scale
Chemists solve solubility problems to produce 100 kg of product
by Carmen Drahl
March 7, 2018
| A version of this story appeared in
Volume 96, Issue 11
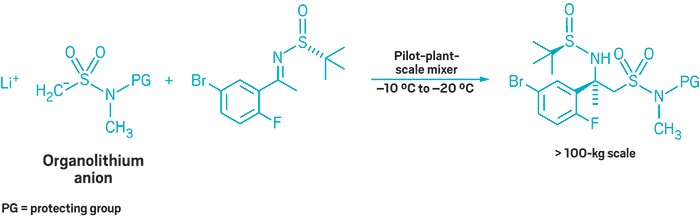
By ferreting out weak points in their production process, Merck chemists have optimized an organometallic flow chemistry reaction at scales in excess of 100 kg (Org. Process Res. Dev. 2018, DOI: 10.1021/acs.oprd.7b00385). Lessons from this work could help others seeking to scale up reactions of their own, the authors say.
The reaction involves adding an organolithium anion to a source of a nitrogen-containing carbon stereocenter. This chemistry builds a crucial chiral center in verubecestat, a compound Merck has evaluated in Phase III clinical trials for treating Alzheimer’s disease. In prior work, David A. Thaisrivongs, John R. Naber, and colleagues decided to run the reaction in flow to increase their yield. Keeping the process flowing kept the product from consuming unreacted starting material.
They had perfected the flow reaction at an approximately 1-kg scale, but going to a pilot plant, and increasing the scale to hundreds of kilograms, introduced unforeseen problems.
A flow reaction works best with homogenous solutions. With longer flow reaction times and larger scales, however, slurries and aggregates started to rear their ugly heads. The researchers came up with fixes for each problem. To eliminate insoluble deposits when generating the organolithium anion, the chemists formed it in batch mode, and then flowed it into the reactor. To prevent starting material from caking on the reaction vessel wall, the team tweaked the order of addition, putting solvent into the system first. Just when they’d had those problems licked, the organolithium anion, which had always been soluble once it was generated, started looking more like a slushy than a solution. The chemists solubilized the anion by adding N,N’-dimethylpropylene urea (DMPU).
“We grew to appreciate that when designing a flow process, it is important to establish robustness, not just for a few minutes in the lab, but over many hours, and at commercial scale,” Thaisrivongs says.
With additional measures to precisely control the temperature of the reaction, the Merck team was able to run the reaction for over three hours at the plant, producing more than 100 kg of product. The future use of this optimized reaction isn’t clear. Investigators last month withdrew verubecestat from a Phase III trial for the second time.
The lessons for flow chemistry, however, still stand. “Handling solids in flow is still a limitation when converting from batch to continuous processes,” says Martin D. Johnson, who works on flow chemistry for Eli Lilly & Co. He calls the work “a notable flow chemistry advance.”
“Flow is not without challenges—sometimes unique ones—when scaling up,” and this group has provided solutions, adds Aaron Beeler, a Boston University chemist and cofounder of the continuous flow technology firm Snapdragon Chemistry.
This article has been translated into Spanish by Divulgame.org and can be found here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter