Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Investment flows at J.P. Morgan Healthcare Conference
Mergers and acquisitions are announced as venture capitalists invest in young companies
by Lisa M. Jarvis
January 11, 2018
| A version of this story appeared in
Volume 96, Issue 3
The J.P. Morgan Healthcare Conference in early January has traditionally been the source of a flurry of acquisition and investment announcements, and this year was no exception. Wheels were not even up on most flights carrying biopharmaceutical executives and investors to San Francisco when capital had begun flowing.
On the event’s first morning, Novo Nordisk proposed acquiring Ablynx, a Belgian antibody drug developer, for about $3.1 billion. In the run-up to the conference, Celgene said it planned to spend up to $7 billion in a staged acquisition of Impact Biomedicine, and Takeda Pharmaceutical unveiled a deal worth roughly $625 million for TiGenix.
Novo wants Ablynx for the antibodies in its development pipeline, including caplacizumab for the treatment of a rare bleeding disorder. Ablynx, however, has rejected Novo’s offer. Ablynx CEO Edwin Moses is not swayed by Novo’s promise that it will continue to invest in Ablynx and keep its R&D base in Ghent, Belgium. Novo is now going public with its offer in an effort to put pressure on Ablynx’s board of directors.
The centerpiece of the Celgene deal is fedratinib, a JAK2 inhibitor that has completed a Phase III study in people with a serious bone marrow disorder called myelofibrosis. The compound was discovered at TargeGen. Sanofi acquired TargeGen in 2010 but later ended development when safety issues arose. The molecule’s inventor, former TargeGen chemist John Hood, started Impact Biomedicine in 2016 to revive it.
Separately, Takeda announced it will buy TiGenix to add a stem cell therapy for Crohn’s disease to its pipeline. The Japanese firm also unveiled a drug discovery pact with Denali Therapeutics, a neuroscience-focused biotech firm formed in 2015 by a group of former Genentech executives.
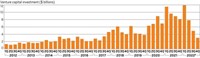
The run-up to the meeting also brought a flood of venture capital to young biotech firms. Last week, venture capitalists laid out more than $650 million in series A funding—a company’s first significant round—to biotech firms working on a range of diseases and technologies.
The lion’s share of that money went to just four companies—BioNTech, Gossamer Bio, KBP Biosciences, and Expansion Therapeutics—building on a trend for venture capitalists to put significant resources into select young firms. Bernard Munos, senior fellow at the Milken Institute’s FasterCures research advocacy center, said he’s “delighted to see capital flowing to drug R&D,” but he cautioned against the risk of breeding inefficiency.
“The large rounds are a concern because having a lot of money in the bank removes the incentive to be creative with drug development,” Munos said, noting that it could discourage biotech firms from pushing boundaries, for example, in how they design trials or collect data.
Investors are also optimistic that the public markets will continue to be favorable for biotech firms preparing to file for initial public offerings (IPOs) of stock. “Everyone is very hopeful the IPO market will remain open for high-quality biotech deals in the queue,” said Wende Hutton, general partner at the venture capital firm Canaan Partners. “There’s a lot of capital in the system. You can’t help but be bullish about the sector right now.”
Speaking on a panel at one of the many side events taking place in San Francisco last week, Atlas Venture partner Bruce Booth agreed that investors are excited about “the huge number of maturing, wonderful biotech companies.” However, the venture capitalist cautioned that a change of political winds during the midterm election cycle could influence the valuations of companies. Booth noted that most companies that went public during the last two months of the 2016 election cycle debuted to “more restrained valuations.”
Start-up spree
The year kicked off with more than $650 million in first-round funding for biotech firms.
| COMPANY | SERIES A FUNDING ($ MILLIONS) | FOCUS |
|---|---|---|
| BioNTech | 270 | mRNA and CAR T cells |
| Gossamer Bio | 100 | In-licensing shelved drug candidates |
| KBP Biosciences | 76 | Medicinal chemistry |
| Expansion Therapeutics | 55 | Small molecules targeting RNA |
| Neurogastrx | 45 | Drugs for gastrointestinal disorders |
| Stoke Therapeutics | 40 | Antisense oligonucleotides |
| Elstar Therapeutics | 39 | Cancer immunotherapy |
| Generation Bio | 25 | Titratable gene therapy |
| NexImmune | 23 | Nanoparticle-based immunotherapies |
Source: companies





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter