Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanosurfactants create droplet-sized reaction flasks
Chemists use magnets, lasers, and electricity to manipulate droplets with nanoparticle skins
by Bethany Halford
January 11, 2018
| A version of this story appeared in
Volume 96, Issue 3

By adding a suit of nanoparticle surfactant armor to otherwise dull droplets, chemists in South Korea have created tiny reaction flasks that they can maneuver and modify using magnets, lasers, and electrical fields. The droplets offer a new way to do microscale chemical reactions that avoids some of the pitfalls associated with microfluidics, such as difficulty mixing solutions and limitations on the types of solvents that can be used.
Bartosz A. Grzybowski, Zhijie Yang, Jingjing Wei, and Yaroslav I. Sobolev, of the Center for Soft & Living Matter at the Institute for Basic Science, create the armored droplets using what they call a nanoparticle surfactant. The surfactant has a nanoparticle core that welds gold to some other nanomaterial, such as Fe3O4. The gold half has a coating of ligands with a polar tail, while the other half is coated with hydrophobic ligands. As a result of these coatings, the particles form monolayers at the interface of organic and aqueous solutions (Nature 2018, DOI: 10.1038/nature25137).
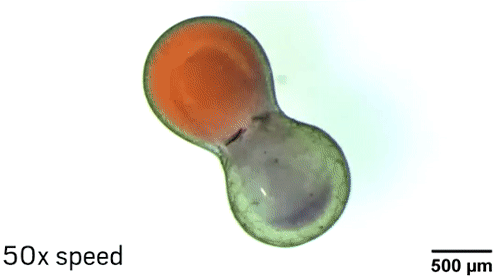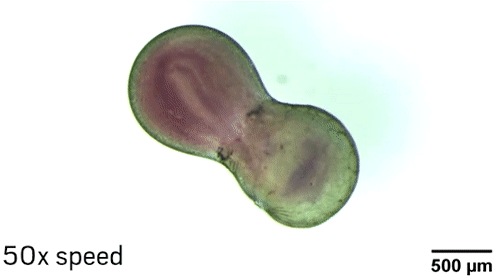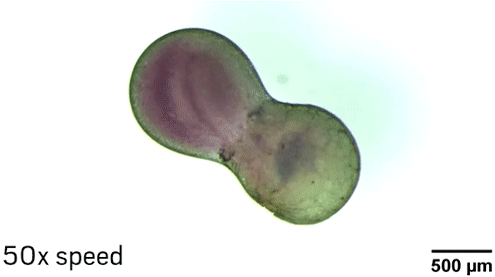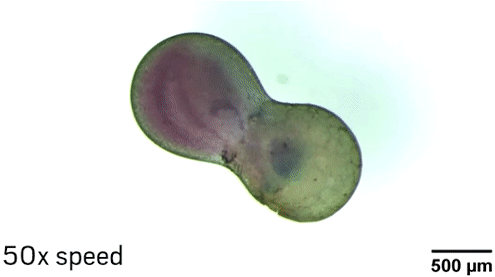

The researchers can manipulate the droplets in different ways thanks to the nanoparticle core of the surfactants. The Fe3O4 makes the particle magnetic, so researchers can move the droplets with a magnetic field. Laser light also makes it possible to position and even spin the droplets because of heating and convection effects near the nanoparticles. “The fluid in the middle of the droplet is not rotating with the same speed as the fluid at the edge of the droplet, so effectively you’re mixing inside the droplet,” Grzybowski explains.
In addition, electrostatic fields can punch tiny openings in the droplets, allowing those that are touching to merge and combine their contents. Grzybowski imagines a microscale chemical factory with hundreds of different droplets that can react with one another. “You zap them with an electric field and you make connections between them.”

To demonstrate the ability of aqueous droplets to serve as reaction flasks, the chemists combined a droplet containing CoCl2 with a droplet containing 1-methylimidazole to create the metal-organic framework ZIF-67.
Grzybowski notes that he was surprised that the thin nanosurfactant skin has such a strong influence on the comparatively larger droplets. It’s impressive, he says, that surfactants measuring just 5–10 nm wide can make a millimeter-sized droplet magnetic or responsive to light.
“This is really neat work,” comments Leroy Cronin, an expert in complex chemical systems at the University of Glasgow. “It shows how to combine electronic, optical, and magnetic control of both individual and large numbers of droplets, as well as shows potential for using them as reaction vessels. I’m excited to see how this is extended later into more complex applications.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter