Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Dimethylcalcium synthesis cracked
Chemists have struggled to make the organometallic compound for decades
by Celia Henry Arnaud
January 24, 2018
| A version of this story appeared in
Volume 96, Issue 5

Dimethylcalcium may sound like a simple compound, but its synthesis has eluded chemists. Researchers reported a synthesis 60 years ago, but no one has reproduced it, casting doubt on whether dimethylcalcium was actually made that first time.
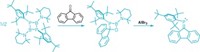
Now, Reiner Anwander and coworkers at Eberhard Karls University of Tübingen have succeeded in synthesizing dimethylcalcium via a metathesis reaction between methyllithium and calcium bis(trimethylsilyl)amide (J. Am. Chem. Soc. 2018, DOI: 10.1021/jacs.7b12984). A synthesis of methylcalcium compounds could lead to new catalysts or reagents for forming carbon-carbon bonds.
“Although methylcalcium compounds have been discussed for more than a century, they’ve always been ill-defined and probably impure,” says Michael S. Hill of the University of Bath. “The isolation and definitive characterization of these compounds is a real synthetic achievement, and the paper as a whole is a striking piece of work.”
The hardest part of making dimethylcalcium turned out to be finding appropriate starting materials. Dimethylmercury might have made a good transmethylating agent, but Anwander forbids the use of it in his lab because of its toxicity. His team turned to methyllithium instead.
But commercial sources of methyllithium are usually contaminated with lithium chloride, which precipitates during the reaction as inseparable lithium and calcium chlorides. Anwander’s team devised a purification method to remove the chloride. By adding potassium bis(trimethylsilyl)amide to a solution of methyllithium in ether, the chemists could trigger the precipitation of KCl. Lithium bis(trimethylsilyl)amide also forms, but it doesn’t interfere with the desired metathesis reaction.
Anwander thinks the methyllithium purification will be useful for the broader chemistry community. “Many people synthesize compounds from methyllithium that contains significant amounts of chloride,” he says. “If such compounds are then used as catalysts, the chloride can influence the chemistry,” he says.
After they made the dimethylcalcium, Anwander’s team used it in a variety of derivatization reactions. For example, they reacted the dimethylcalcium with calcium iodide to make a methylcalcium iodide-heavy Grignard reagent. By reacting dimethylcalcium with a bulky ligand, they were able to make complexes with a terminal calcium methyl. Anwander wants to test whether the terminal calcium methyl will make a good catalyst.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter