Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Method detects widespread histidine phosphorylation in bacterium
Protein modification might be more common than previously thought
by Celia Henry Arnaud
February 5, 2018
| A version of this story appeared in
Volume 96, Issue 6
Protein phosphorylation helps control most processes in cells. Phosphorylation most often occurs at serine, threonine, or tyrosine residues in proteins. Histidine residues also can be phosphorylated, but biologists have assumed this is rare. A new study suggests that histidine phosphorylation might be more common than previously thought.
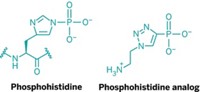
Albert J. R. Heck, Simone Lemeer, and coworkers at the University of Utrecht have developed a new proteomics approach for analyzing histidine phosphorylation (Nat. Methods 2018, DOI: 10.1038/nmeth.4580). They use Fe3+-immobilized metal affinity chromatography to enrich peptides with phosphorylated histidines in samples and then analyze the peptides by liquid chromatography-tandem mass spectrometry. By applying milder conditions than are typically used in phosphopeptide enrichments, the team can prevent hydrolysis of the unstable histidine phosphorylation.
The researchers demonstrated the power of the method by studying protein histidine phosphorylation in Escherichia coli. They detected 2,129 phosphorylation sites, which is about 10 times as many as seen with established analytical methods. Of those sites, 246 were phosphohistidine sites, occurring on 173 bacterial proteins. The researchers were surprised to find that histidine phosphorylation represented about 10% of the overall E. coli phosphoproteome, making it about as abundant and frequent as phosphorylation on threonine or tyrosine residues.
The researchers think that the new method “could help answer long-standing questions about whether histidine phosphorylation also plays a major biological role in higher organisms,” including humans.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter