Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Sidestepping white phosphorus
Compounds previously made from toxic and pyrophoric elemental phosphorus can now be prepared starting with phosphoric acid
by Bethany Halford
February 12, 2018
| A version of this story appeared in
Volume 96, Issue 7

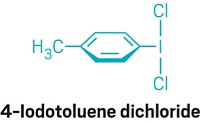
Making phosphorus-containing compounds is not something to be undertaken lightly. Typically, phosphate is reduced to white phosphorus—a pyrophoric and toxic allotrope of the element—which is then reacted with chlorine gas to produce phosphorus trichloride—a base reagent for making phosphorus-containing compounds. Despite the dangerous reagents, this route is used to mass-produce compounds such as the herbicide glyphosate and the battery electrolyte lithium hexafluorophosphate. Now, MIT chemists Michael B. Geeson and Christopher C. Cummins report an alternative route to organophosphorus compounds and other phosphorus-containing chemicals that avoids using white phosphorus as an intermediate (Science 2018, DOI: 10.1126/science.aar6620). The reaction begins with dehydration of phosphoric acid by sodium chloride to produce trimetaphosphate. The MIT chemists then reduce the trimetaphosphate with trichlorosilane—a chemical made in large amounts for the semiconductor industry—to produce the previously unknown bis(trichlorosilyl)phosphide anion. This anion reacts with various compounds to produce organophosphorus compounds (example shown), phosphine gas, and the hexafluorophosphate anion. Geeson and Cummins propose the route could be adopted to replace syntheses of critical chemicals that rely on white phosphorus, if production of the material should cease.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter