Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Proteins don’t need well-defined structures to bind strongly
Two proteins bind with high affinity in a complex that changes form continuously
by Stu Borman
February 21, 2018
| A version of this story appeared in
Volume 96, Issue 9

Biochemists have long thought that biomolecules must have or develop well-defined structures to bind one another. But an unexpected discovery smashes that conventional wisdom. Researchers have found two proteins that bind strongly yet remain unstructured in a complex that changes form continuously.
Intrinsically disordered proteins aren’t just quirky weirdos in cells. Many human proteins have long disordered regions, and many disordered proteins play key roles in normal cellular function and disease. The new type of interaction, though surprising, could be widespread in human cells, and its discovery has potential implications for designing disordered protein-based drugs and nanotechnologies, the researchers say.
The idea that two proteins could form a high-affinity complex while undergoing highly dynamic conformational changes has been “nearly heretical,” says Richard W. Kriwacki of St. Jude Children’s Research Hospital, who was not involved in the new study. The beauty of the work is its rigorous use of structural and modeling techniques to demonstrate the new type of interaction, he says.
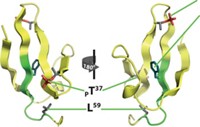
In the study, Benjamin Schuler and Alessandro Borgia of the University of Zurich, Birthe B. Kragelund of the University of Copenhagen, Robert B. Best of the National Institutes of Health, and coworkers observed the interactions between histone H1 and prothymosin-α (ProTα). Histone H1 is highly positively charged and supports the structure of chromatin, the cellular material that packages DNA in the nucleus. ProTα is a strongly negatively charged chaperone that helps regulate histone H1’s interactions with chromatin. Both are intrinsically disordered, except for a small, folded region on histone H1.
The team determined that the pair binds strongly with picomolar affinity. Using structural analysis techniques such as single-molecule fluorescence, fluorescence correlation, nuclear magnetic spectroscopy, and circular dichroism, along with computer simulations, the researchers found that the bound proteins remain disordered and dance around each other on a 100-ns timescale in a highly dynamic but very tight embrace (Nature 2018, DOI: 10.1038/nature25762).
The sizable electrostatic interaction between the two proteins obviates the need for a single defined binding site, the scientists conclude. But this type of interaction isn’t likely to be highly selective, so the researchers speculate that cellular systems localize or simultaneously express the pair so the proteins can find each other and bind.

The experimental and theoretical techniques the group used “set the stage for the investigation of other complexes of this type,” Schuler says. Similar interactions may be widespread in the human proteome, as hundreds of proteins that are likely intrinsically disordered are also highly charged.
“What is new is evidence from several independent lines of investigation that the interaction has no specific anchoring sites and yet is characterized by extremely high affinity,” says Sonia Longhi of the National Center for Scientific Research and Aix-Marseille University.
“What makes this so exciting is that it is the only example of a discrete complex of two disordered proteins interacting with each other without taking on ordered structure,” adds Julie D. Forman-Kay of the Hospital for Sick Children in Toronto. However, she notes that disordered proteins have been shown to interact without structural order in large-scale associated states—ensembles of proteins such as those that form small compartments in eukaryotic cells.
This article has been translated into Spanish by Divulgame.org and can be found here.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter