Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Researchers settle long-standing debate on chromosome-compacting complex
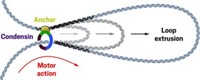
Condensin maintains chromosome structure by acting as a molecular motor to extrude DNA loops
by Celia Henry Arnaud
February 23, 2018

A protein complex known as condensin is one of a family of complexes responsible for organizing and compacting chromosomes so they fit in cells. Condensin’s specific job is organizing chromosomes so they can segregate properly during cell division. Some researchers have proposed that these complexes work by extruding DNA in loops. But scientists have never directly observed this extrusion.
Cees Dekker of Delft University of Technology, Christian H. Haering of the European Molecular Biology Laboratory, and coworkers have now used fluorescence microscopy to watch condensin make DNA loops (Science 2018, DOI: 10.1126/science.aar7831).
Condensin is a ring-shaped complex of five proteins. The mechanism by which it works has been a subject of debate in chromosome biology for decades. One school of thought suggests that condensin just clamps existing DNA loops together. The other camp proposes that condensin is actually a molecular motor that extrudes the DNA loops itself. Another question has been how many complexes are involved—just one or more than one?
In the new study, the researchers anchored both ends of a long piece of fluorescently labeled double-stranded DNA to a surface. They added fluorescently labeled condensin and adenosine triphosphate as an energy source and applied a constant flow of buffer solution to help stretch out the DNA.
“We see a condensin land on the DNA and start to extrude a loop,” Dekker says. Simultaneous imaging of both the DNA and protein complex demonstrated that a single condensin complex at the base of the loop reels in DNA asymmetrically: One side of the ring holds onto the strand while the other pulls the DNA through.
“For many years, I was very critical of this idea that condensin could work as a DNA extrusion motor,” Haering says. “But now that we can see it, I think there’s no doubt about it.”
Leonid Mirny of Massachusetts Institute of Technology and coworkers recently proposed that loop extrusion is the main mechanism of chromosome compaction during one part of the cell cycle called interphase (bioRxiv 2018, DOI: 10.1101/264648), as well as during cell division (Science 2018, DOI: 10.1126/science.aao6135). “We are very excited to see direct confirmation of our loop extrusion hypothesis,” he says. Other studies, he says, have provided supporting evidence, “but all those studies were missing direct visualization of loop extrusion.”
Frank Uhlmann, a researcher at the Francis Crick Institute who studies mechanisms of chromosome segregation, likewise calls the finding exciting. But he still has questions.
“The field has almost complete knowledge of condensin’s molecular structure, but the known ATP binding site and the candidate DNA binding regions don’t add up to a plausible mechanism for condensin moving along the DNA,” Uhlmann says. He wonders whether condensin really moves along the DNA by itself or if it needs another enzyme to help it move.
Camilla Björkegren (formerly Sjögren), who studies chromosome biology at the Karolinska Institute, finds the work beautiful, but she too has questions. “This is naked DNA. What will it look like in the context of chromosomes,” which contain histone proteins and other components of structures called nucleosomes?
Those are the kinds of questions that Dekker and Haering plan to focus on next. “When we look at the architecture of the condensin complex, it’s definitely not clear how this can work as a DNA motor,” Haering says. Dekker adds, “That will be the question for the next few years that we will try to answer.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter