Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalyst makes acetic acid from methane
Isolated metal ions drive coupling chemistry at low temperature
by Mitch Jacoby
March 19, 2018

By dotting the pores of a zeolite with individual rhodium atoms, researchers have come up with a catalyst that couples methane, carbon monoxide, and oxygen to produce acetic acid at low temperature. The findings, which the team reported Sunday at the American Chemical Society national meeting in New Orleans, could lead to a low-cost, low-energy catalytic process for manufacturing the organic acid.
The chemical industry produced about 14 million metric tons of acetic acid worldwide in 2017 for use as a reagent to make monomers and other compounds, as well as for use in the medical and food industries.
Manufacturers produce acetic acid through a number of commercial processes including methanol carbonylation, which couples CO to the alcohol. Methanol is typically produced from a mixture of CO and hydrogen known as synthesis gas, which in turn is made from coal or methane. Some of those industrial processes run at temperatures as high as 1,000 °C.
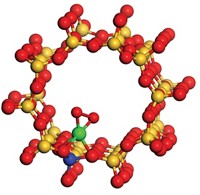
Speaking in a symposium organized by the Division of Catalysis Science & Technology, Franklin (Feng) Tao of the University of Kansas reported that he and his coworkers used a wet chemical process and ion-exchange chemistry to deposit isolated rhodium cations inside the micropores of ZSM-5, an aluminosilicate zeolite. Based on X-ray absorption spectroscopy and other methods, the team concluded that the catalyst takes the form of anchored RhO5 species.
The team evaluated catalysts with a range of rhodium concentrations by first dispersing them in water and then delivering the suspensions to a reactor charged with methane, CO, and oxygen at various partial pressures. The reactions produced a mixture of acetic acid, formic acid, and methanol. By optimizing the conditions, the team boosted the selectivity for acetic acid to 70% at just 150 °C. Under those conditions, the anchored catalyst was more than 1,000 times as active as solution-phase rhodium cations.
To understand how the catalyst does its job, the team used isotope-labeling and other experimental methods coupled with computational analysis. The researchers proposed a complex 18-step mechanism, which generated a lively discussion at the presentation.
“The mechanism is quite convincing and seems consistent with the data,” remarked Aditya (Ashi) Savara, a symposium co-organizer and catalysis specialist at Oak Ridge National Laboratory. “The selectivities, activities, and reaction rates under those conditions, is surprising and impressive.”
Georgia Tech’s Meilin Liu also praised the study, noting that rationally designed catalysts like the ones described by Tao may benefit other catalytic processes by considerably reducing energy consumption and cost.
CORRECTION: This story was changed on March 22, 2018, to update the scale of global acetic acid production from the 2003 level of about 6.5 million metric tons to the amount made in 2017, about 14 million metric tons. The unit of measure of this value was also updated from tons to metric tons.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter