Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Antifungal analog offers reduced toxicity
Improved version of amphotericin B is now in clinical trial, but debate continues about its mechanism of action
by Mark Peplow, special to C&EN
November 9, 2023

By tweaking the structure of a powerful antifungal drug called amphotericin B (AmB), chemists have eliminated its toxicity without reducing its potency (Nature 2023, DOI: 10.1038/s41586-023-06710-4). Fungal infections kill more than one million people every year, so the modified drug has the potential to be a lifesaver, and is now being evaluated in a phase 1 clinical trial.
“People have been trying to make amphotericin non-toxic for more than seventy years,” says Martin D. Burke of the University of Illinois at Urbana-Champaign, who co-led the research with Chad M. Rienstra of the University of Wisconsin–Madison. Although AmB is one of the most powerful antifungal medicines, its severe side effects—particularly in the kidneys—limit its use.
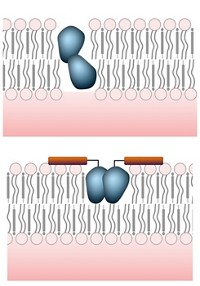
There is also intense debate about AmB’s mechanism of action. AmB can create pores in fugal cell membranes, and many scientists think this allows the cell’s innards to leak out, killing the cell. But over the past decade, Burke and colleagues have argued that these pores are not the primary cause of AmB’s antifungal effect. They say that AmB can also form a sponge-like assembly outside the fungal cell, which extracts ergosterol from the membrane. Removing this crucial membrane component is responsible for killing the cell, Burke says, possibly because the loss of ergosterol impairs the function of membrane proteins.

He and his colleague have now explored this “sterol sponge” mechanism in more detail, using solid-state nuclear magnetic resonance (NMR) to study the complex formed by AmB and ergosterol. They also found that AmB forms similar complexes with cholesterol, found in human cell membranes, suggesting that the same sponge mechanism may explain how the drug damages kidney cells.
This gave the team vital clues about how modifying AmB’s structure could reduce its toxicity. The drug is a polyene, with a large ring containing a series of carbon-carbon double bonds; it also bears a carboxylic acid group, and an amino sugar called mycosamine. NMR data showed that AmB could bind both ergosterol and cholesterol through a hydrogen-bonding network involving a hydroxyl group on mycosamine. Ergosterol also felt additional binding from AmB’s polyene section.
These NMR insights led the researchers to create an analog of AmB in which they flipped the orientation of the mycosamine hydroxyl group. This disrupted the hydrogen-bonding network and dramatically reduced the analog’s ability to bind sterols, making it much less toxic according to tests in cells and mice. However, it was also less potent than AmB.
In another analog, the researchers converted AmB’s carboxylic acid into an amide, enabling it to suck up ergosterol more quickly than unaltered AmB.
Finally, they combined both of these changes in a single molecule called AM-2-19, which is very good at binding ergosterol, but does not form a complex with cholesterol. This made AM-2-19 at least as potent as AmB against more than 500 different species of fungi—including some that were resistant to AmB—yet nontoxic to human cells. Tests in mice with fungal infections confirmed that AM-2-19 is highly effective without causing renal problems. “It’s one of the best amphotericin B analogs every reported,” says Michio Murata of Osaka University, who works on AmB and was not involved in the research.
The work is “groundbreaking”, adds Mariusz Gagoś of Maria Curie-Skłodowska University, who also studies AmB. “For many years, people have been looking for AmB with less toxicity and unchanged antifungal activity.”
Despite those plaudits, Murata and Gagoś both say they are still unconvinced by the sterol sponge mechanism. Murata points out that the team’s NMR experiments used carefully prepared complexes that may not reflect the messy reality of the cell membrane environment.
As the debate continues, Burke’s spin-out company, Sfunga Therapeutics, is already dosing volunteers with AM-2-19 in a phase 1 trial. Meanwhile, he and Rienstra think that other polyene natural products may operate through similar sponge mechanisms, and their teams are now investigating whether such compounds can be modified to enhance potency or decrease toxicity. “There’s a quite a large number of these that we could explore further,” Rienstra says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter