Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Antidepressant Binding Findings
Structural Biology: Four drug types bind in a similar way, but nonhuman model raises questions
by Celia Henry Arnaud
October 21, 2013
| A version of this story appeared in
Volume 91, Issue 42

Antidepressants target transporter proteins in the human brain that regulate the levels of neurotransmitters such as serotonin and dopamine. The drugs block the transporters from removing neurotransmitters from nerve-cell synapses, thus having a psychoactive effect. But because these human proteins have been difficult to crystallize, information about the exact way they bind to the drugs has been limited.
New findings reported on Oct. 13 suggest that four types of antidepressants bind with transporter proteins in a similar way (Nature 2013, DOI: 10.1038/nature12648).
Although the discovery of a common binding scheme for antidepressants drew excitement from some quarters, skepticism
In the study, Eric Gouaux and coworkers at the Vollum Institute at Oregon Health & Science University solved 12 high-resolution crystal structures of a bacterial mimic of biogenic amine transporters complexed with four types of antidepressants. They used the modified bacterial transporter because multiple attempts to crystallize human transporters have been unsuccessful.
To create the model, they genetically engineered a bacterial transporter called LeuT by adding amino acids from the human serotonin transporter binding site. They believe the modified transporter, called LeuBAT, binds antidepressants in the same way that human biogenic amine transporters do.
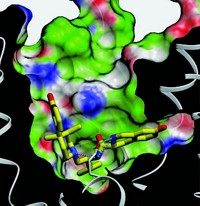
Gouaux and colleagues’ study shows that antidepressants bind LeuBAT in the transporter’s main binding pocket—not, as earlier studies had indicated, at an “extracellular vestibule” site 15 Å away from the main binding site. The drugs have a saddlelike shape that straddles one of the transmembrane helices and wedges between two other pairs of transmembrane helices, locking the transporter in an inactive open conformation, Gouaux says.
“The results will no doubt have those who have pursued the structural basis of serotonin transporter pharmacology buzzing—that is, excited by the effort, but also wondering how well the structures relate to the real thing,” says Randy D. Blakely, a professor of pharmacology and psychiatry at Vanderbilt University School of Medicine.
Gouaux acknowledges that there could be doubts about whether the mutated bacterial transporter is an accurate model for eukaryotic, including human, transporters. However, a structure of an antidepressant bound to an insect transporter that his group published last month shows similar binding to LeuBAT, boosting confidence in the modified bacterial transporter finding (Nature 2013, DOI:10.1038/nature12533). The two studies “provide a mutually reinforcing view of how these inhibitors act,” Gouaux says.
“These two papers represent seminal work in the area of neurotransmitter transporters and mechanism of action of antidepressants,” says Kristian Strømgaard, a professor of chemical biology in the department of drug design and pharmacology at the University of Copenhagen. Nevertheless, he notes that a “primary caveat is that LeuBAT is not functional. Thus, the work provides seminal information on binding of antidepressants and fits well with previous modeling and pharmacological studies but provides less information on function.”
Blakely agrees that LeuBAT doesn’t actually work as a neurotransmitter transporter, and he points out that its selectivity for antidepressants seems to be a hybrid of several neurotransmitter transporters’ selectivities. This raises questions about whether its binding mechanism is a valid representation of the way antidepressants bind mammalian transporters, he says.
But Gouaux and coworkers believe theirs is an improvement over existing models. “This work will go a long way to pushing the field toward more reliable and more realistic models,” Gouaux says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter