Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Researchers design allosteric protein from scratch
Complex protein combo sets a new bar in protein engineering
by Celia Henry Arnaud
December 17, 2020
| A version of this story appeared in
Volume 98, Issue 48
In a milestone for protein engineering, researchers have designed from scratch a protein that exhibits allosteric regulation. With allostery, binding at one site of a protein affects binding or activity at a distant second site. This finding opens the door to designing controllable biological catalysts.
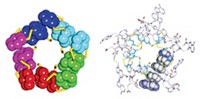
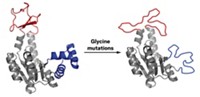
As a proof of concept, Marco Chino and Angela Lombardi of the University of Naples Federico II, William F. DeGrado of the University of California, San Francisco, and coworkers combined two previously designed proteins with different functions into a single protein that links these functions (Proc. Natl. Acad. Sci. U.S.A. 2020, DOI: 10.1073/pnas.2017062117). Each protein consists of a bundle of four α-helices. One protein binds a zinc-porphyrin, whereas the other catalyzes phenol oxidation.
Guided by computer simulations, the researchers stacked the proteins on top of each other, connecting them via four linkages—a complex arrangement that ensured a tight connection between the two functional parts of the new protein. The use of four linkers is a first for protein design, Chino says. When one domain binds a Zn-porphyrin, its structure shifts, slowing down the oxidation reaction in the other domain, the researchers found.
Advertisement
They hope they can further tune catalysis in future iterations by taking advantage of porphyrin’s light absorption. By bringing the functional sites closer to each other, it should be possible to further change the oxidation reaction rate by shining light on the bound porphyrin, DeGrado says.
Allostery is “hard even to predict in natural proteins and much harder to design,” Aitziber L. Cortajarena, who designs protein building blocks at the Center for Cooperative Research in Biomaterials–CIC biomaGUNE, writes in an email, calling the work “very interesting and significant.” The research will create opportunities for applications using allostery for responsive regulation, according to Cortajarena.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter