Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Scientists sniff out the structure of a human olfactory receptor
The research gives a first look at how chemicals trigger our sense of smell
by Shi En Kim
March 23, 2023
| A version of this story appeared in
Volume 101, Issue 10

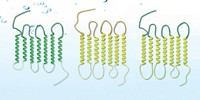
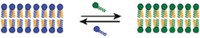
Our noses encounter a vast diversity of chemicals every day, but scientists know little of how odorants stir awake the receptors in our bodies. A recent study is the first to parse the structure of a human olfactory receptor, called OR51E2 (Nature 2023, DOI: 10.1038/s41586-023-05798-y). The study’s authors reveal the full molecular picture of how OR51E2 interacts with propionate, the pungent player behind Swiss cheese.
“It’s a landmark paper,” says Vanessa Ruta, a Rockefeller University sensory neuroscientist not involved in the study.
Our bodies detect environmental stimuli with cellular sentinels called G-protein- coupled receptors (GPCRs). Of the 800 GPCRs encoded by the human genome, roughly half are tied to smell.
Smell receptors are notoriously tricky to produce in sufficient quantities for structure studies. According to corresponding author and biochemist Aashish Manglik of the University of California, San Francisco, his team homed in on OR51E2 because it moonlights in the kidney and gut outside the nose, hinting that it is atypically stable. As OR51E2 is geared toward water-soluble compounds, the researchers chose the carboxylic acid propionate as the odorant.
The scientists used cryo-electron microscopy to elucidate OR51E2’s 3D structure then worked out its chemical interactions via computer simulations. They found that propionate latches onto an “on” switch, the arginine in the receptor’s binding pocket, via ionic and hydrogen bonds.
OR51E2 is a low-hanging fruit; other olfactory receptors will be harder to crack. Manglik’s team plans to apply protein engineering to create stabler versions of the receptors that are easier to study. Tweaking the structure of OR51E2 to predict other receptors’ shapes would also be a good place to start, Manglik says.
CORRECTION:
This article was updated on April 6, 2023, to correct the year the study was published. It was 2023, not 2022.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter