Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Alder-ene-catalyzing enzymes discovered
Fungal enzymes could be used to make compounds with substituted pentenes
by Celia Henry Arnaud
October 1, 2020
| A version of this story appeared in
Volume 98, Issue 38

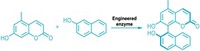
Pericyclic reactions, which involve concerted electron movement and a cyclic transition state, have long been a part of the synthetic chemist’s tool box. But finding enzymes that catalyze such reactions, particularly a class called Alder-ene reactions, has been difficult. A research team has now found Alder-ene enzymes that might be used as biocatalysts to more efficiently make compounds that contain substituted pentenes (Nature 2020, DOI: 10.1038/s41586-020-2743-5).
“We believe that a lot of the synthetic reactions that have been invented have long existed in nature, and we just haven’t found them yet,” says Yi Tang, who led the research along with University of California, Los Angeles, colleague Kendall Houk and Jiahai Zhou of the Shanghai Institute of Organic Chemistry.
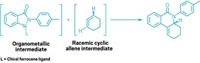
The researchers found candidate enzymes in several species of fungi. Starting from the same alcohol-containing intermediate, one group of enzymes formed the hetero-Diels-Alder product, an oxygen-containing heterocycle. But another group, exemplified by an enzyme called PdxI, formed a substituted pentene, the Alder-ene product.
PdxI’s formation of the substituted pentene is a sign of high periselectivity, the ability to select one pericyclic reaction over another. The team used crystal structures and molecular simulations to determine why. “PdxI positions the substrate so that only the Alder-ene transition state can be accessed,” Tang says. In protein engineering studies, the researchers found that they could switch the periselectivity of PdxI from the Alder-ene product to the hetero-Diels-Alder product by changing a single amino acid in the active site.
The work “is an impressive combination of biochemistry, structural biology, and modeling to understand and manipulate the reaction coordinate of pericyclic enzymes,” Tobias J. Erb of the Max Planck Institute for Terrestrial Microbiology, who also studies pericyclases, says via email. “Although the authors did not demonstrate full control with their engineering efforts, they show that it is in principle possible to shift pericyclic selectivity between hetero-Diels-Alder and Alder-ene reactions.”
The researchers on the new study anticipate that these or related enzymes could find use as biocatalysts for synthetic chemistry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter