Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biotechnology
Tobacco-grown COVID-19 vaccine moves ahead
by Lisa M. Jarvis
March 20, 2021
| A version of this story appeared in
Volume 99, Issue 10
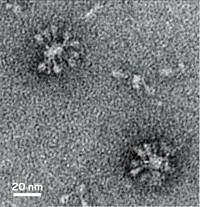
Medicago and GlaxoSmithKline have begun a Phase 3 study of Medicago’s COVID-19 vaccine, which comprises recombinant spike protein derived from plants and an adjuvant developed by GSK. The companies aim to enroll 30,000 people in the placebo-controlled trial of a two-dose vaccine regimen. Medicago exposes tobacco plants to a plant-specific bacterial vector that encodes for a gene sequence of choice—in this case the key protein used by SARS-CoV-2 to enter human cells. The plants spent several days making viruslike particles, which are extracted and purified.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter