Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Epigenetics
New histone modification identified
Common food preservative might be source of lysine benzoylation
by Celia Henry Arnaud
August 30, 2018
| A version of this story appeared in
Volume 96, Issue 35

Posttranslational modifications on histone proteins play a role in regulating chromatin structure and gene expression. Now another so-called histone mark can be added to the list: lysine benzoylation.
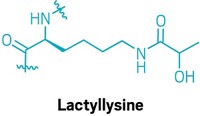
Yingming Zhao of the University of Chicago and colleagues discovered the mark through a proteomic analysis of histones extracted from human liver cancer cells and mouse leukemia cells. The team found 22 benzoylated lysines (structure shown), most of which were located near the histones’ N-terminal tails (Nat. Commun. 2018, DOI: 10.1038/s41467-018-055567).
A likely source of the benzoyl group is sodium benzoate, which is a common food preservative. When the researchers treated cells with the chemical, they observed a dose-dependent increase in the amount of benzoyl-coenzyme A, which they believe transfers the benzoyl group to lysine. The team also found that benzoylation turned on multiple gene pathways.
They don’t know what enzymes are responsible for benzoylating lysine. But they did find that the enzyme SIRT2, a member of the histone deacetylase family, can remove the histone mark.
“The finding that benzoate can end up on histones is very interesting, especially given that sodium benzoate is a common chemical food preservative,” says Hening Lin, who studies epigenetic modifications at Cornell University. More work is needed, Lin says, to determine the physiological importance of this modification.
Zhao’s team next plans to examine whether the modification is associated with any diseases. They also want to dissect how the modification is regulated by looking for the enzymes that add and remove it, the proteins that receive it, and those that bind it.
CORRECTION:
This story was updated on Sept. 5, 2018, to correct the structure of benzoylated lysine. The original structure mistakenly depicted benzoylated ornithine.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter