Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Infectious disease
Cationic peptides show antibacterial promise
New research looks at the membrane-disrupting biochemistry of novel small molecules
by Benjamin Plackett, special to C&EN
July 20, 2023
| A version of this story appeared in
Volume 101, Issue 24

Alexander Fleming cautioned in his 1945 Nobel Prize lecture that antibiotic resistance was inevitable. His warnings have never seemed more prescient; antibiotic-resistant infections killed approximately 1.3 million people in 2019. Now, scientists have described a novel approach to circumvent resistance, using short cationic peptides to kill bacteria (ACS Infect. Dis. 2023, DOI: 10.1021/acsinfecdis.3c00238).
The researchers decided to investigate the bacteria-quelling capacity of peptides because previous work has shown that natural peptides produced by various organisms can have antimicrobial activity. But, peptides are easily attenuated by enzymes, which makes their durability a concern.
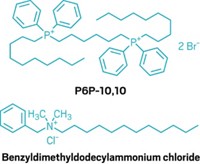
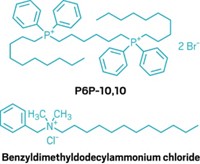
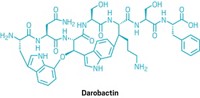
The authors of the new study sought to address this limitation by adding unnatural cationic amino acids to peptide sequences. In doing so, they created four novel short cationic peptides—P1, P2, P3 and P4—which they hoped would be more resistant to enzymes.
“We believe that the future development of peptide drugs will continue to build on the strengths of naturally occurring peptides with the application of rational design to improve their weakness,” says Rubina Chowdhary, one of the study’s authors and a researcher at the Indian Institute of Integrative Medicine.
Chowdhary and her colleagues tested these peptides by growing 12 different strains of bacteria, including Escherichia coli and methicillin-resistant Staphylococcus aureus, and measuring the concentration of peptides needed to kill the pathogens. One of the peptides stood out. “P3 was by far the most promising,” says Katharina Richter, a biomedical researcher at the University of Adelaide who wasn’t involved in the research.
P3’s kill kinetics—the length of time it takes for a compound to kill a bacterium—was also impressive. “A dose of 4 mg/ml kills the whole population of some species within 4 hours and that’s a feasible dose for human applications,” Richter says.
Chowdhary also conducted similar experiments with biofilms, communities of bacteria living within a slimy matrix of their own making. “Biofilms can be up to 1,000-fold more resistant to compounds,” Richter says. If a new approach doesn’t work against biofilms, “there’s no chance it will ever make it to clinical operation.”
Finally, the researchers produced fluorescent micrographs, which showed that P3 punctured the bacterial membranes—a potential mechanism of action. Chowdhary says that she and her colleagues want to test their findings in animal studies next.
Richter says they should also broaden the types of bacteria they investigate. “They tested a range of species but only a single strain within a species,” she says. “It would be good to see if P3 works as well for different strains of bacteria, in particular strains cultured from patients.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter