Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Infectious disease
NMR method allows scientists to study how viruses bind sugars
New technique provides quantitative information about how SARS-CoV-2 protein binds cell surface sugars
by Laura Howes
July 7, 2022

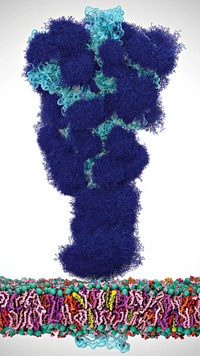
A group of researchers led by Ben Davis of University of Oxford and the Rosalind Franklin Institute has developed a nuclear magnetic resonance (NMR) technique to study the molecular interactions between pathogens and the cells they infect. In a demonstration of the new approach, the researchers found that the original strain of SARS-CoV-2 can latch on to sugars on human cells, possibly helping the virus bind and enter (Science 2022, DOI: 10.1126/science.abm3125).
Sugars, as Davis says, are “always there.” They play multiple roles in biological systems. One key function of sugars is helping different biological entities recognize each other. At the beginning of the pandemic, Davis wondered if sugars might play a role in how SARS-CoV-2 enters human cells. Scientists had determined that the virus can enter human cells when the spike protein on its surface interacts with the ACE2 receptor on cells, but Davis and his team went looking for a complementary mechanism involving sugars on the surface of human cells.
The SARS-CoV-2 spike protein is covered in its own sugars, or glycans. These glycans shield the spike and help it rearrange during infection. But the sheer number of these glycans makes it too complicated for existing techniques to detect if the spike protein also binds sugars on cell surfaces.
To solve this puzzle, the team expanded on an existing NMR technique. In the new version, both the protein and sugar are magnetized in an NMR machine and then just the protein gets excited by radio frequency pulses. If the sugar and protein are bound, some of this excitation will transfer between the molecules. By comparing the NMR spectra produced at different points during this process, researchers can determine physical constants such as the binding strength.
The team combined these results with high-resolution imaging to show that in the original strain of the SARS-CoV-2 virus, there is a binding pocket in the spike protein for a sugar called sialic acid. The team used cryo-electron microscopy to confirm their findings.
The new technique is “definitely a game-changing method,” says glyco-modeller Elisa Fadda of Maynooth University. She is excited that the method provides quantified data about the interaction between viral proteins and cell surface sugars. “It’s definitely something we need,” she adds.
Davis suggests that sugar-binding could be the key to the jump the virus made from animals to people. Sugars may also explain differences in disease severity among patientsts at the beginning of the pandemic, he adds, because the sugars on some patients’ cells may have made it easier for the virus to infect, compared with the sugars on other people’s cells. Similar sugar binding sites exist on the spike proteins of related coronaviruses like the one that causes Middle East Respiratory Syndrome (MERS), but the binding pocket has disappeared in subsequent variants of SARS-CoV-2.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter