Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microbiome
Fluorescent probe brings toxin linked to colon cancer to light
Method rapidly reveals gut bacteria producing high colibactin present in tissue samples
by Cici Zhang, special to C&EN
July 17, 2019
| A version of this story appeared in
Volume 97, Issue 29

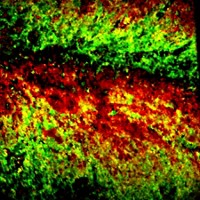
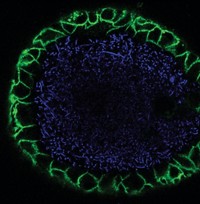
Over the last decade, cancer researchers have learned that a highly reactive, DNA-damaging toxin called colibactin could mark the onset of colon cancer. Certain strains of Escherichia coli in the gut produce the genotoxin, and up to 67% of people with colon cancer harbor such strains, compared with only 20% of those without the disease. But scientists still don’t have a full picture of colibactin’s structure or its mechanism of action because no one has managed to isolate the compound. To make isolation easier, a team led by Kenji Watanabe of the University of Shizuoka has developed a fluorescent probe that allows fast, high-throughput screening of colibactin-producing bacteria and identification of high-producing strains (Org. Lett.2019, DOI: 10.1021/acs.orglett.9b01345). With such strains in hand, scientists could have a better shot at isolating the compound and determining its complete structure, the researchers say.
The fluorescent probe works by detecting the activity of ClbP, a peptidase that is part of the final steps of colibactin synthesis. ClbP removes a protective group, N-myristoyl-D-asparagine (N-myr-Asn), from the colibactin precursor, turning the molecule into the active genotoxin, which then gets secreted out of the E. coli cells. Inspired by this process, researchers made their probe by attaching a fluorescent tag to N-myr-Asn and then delivered it to cultures of individual bacterial strains. When active ClbP is present, the enzyme recognizes its N-myr-Asn target and cleaves the probe’s fluorescent tag, resulting in an increase in fluorescence. From this signal, researchers can infer which bacterial strains have ClbP activity and therefore are producing colibactin.
Watanabe and coworkers tested the probe with colon cancer tissue samples in 96-well plates. The probe revealed the presence of colibactin-producing bacteria in the samples as accurately as polymerase chain reaction (PCR), the gold standard test. They also showed that higher fluorescence intensities corresponded with higher colibactin production levels in E. coli strains. One high-producing strain identified by the probe made 26 times as much colibactin as a strain commonly used as a positive control.
The probe could also help screen human stool samples directly to identify colibactin-producing strains, even before cancer develops, Watanabe says. Compared with conventional identification methods, such as PCR and liquid chromatography/mass spectrometry, this probe-based fluorescent assay is cheap and takes only a few hours, he adds.
Watanabe’s team is not alone in developing probes to find colibactin-producing bacteria. A group led by Harvard University’s Emily Balskus, whose team revealed that colibactin damages DNA in the gut through alkylation, recently reported a similar fluorescence-tagged ClbP probe that they tested in vitro (ACS Chem. Biol. 2019, DOI: 10.1021/acschembio.9b00069). Matthew Volpe, a graduate student who led the work, says it’s exciting to see the two studies complement each other. He adds that the activity difference between strains identified using the Watanabe group’s probe is “quite remarkable.” The next critical question, he says, is whether the high-producing E. colistrains found in this study are actually more genotoxic than other strains when they are in the human gut.
Earlier this year, Watanabe established a company called Adenoprevent that aims to offer screening services to people who want to know whether they host colibactin-producing E. coli, a high-risk indicator for colon cancer. Watanabe says he aims to test 18 million samples a year by 2025.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter