Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Natural Products
Chemists devise efficient route to garlic compound
Biological studies of ajoene and analogs may reveal more details about the seasoning’s antimicrobial activity
by Katharine Sanderson, special to C&EN
August 29, 2018
| A version of this story appeared in
Volume 96, Issue 35
Garlic’s power lies beyond merely pepping up a curry or bowl of pasta. It also contains potentially potent antibacterial and antimicrobial compounds, among them an organosulfur compound called ajoene.
However, obtaining large quantities of ajoene for biological testing isn’t as simple as grabbing a huge garlic press; just 0.1%–0.5% of garlic is ajoene, and purifying it takes a lot of work. Making ajoene from another garlic component—allicin—is an alternative, but the route suffers from low yields.
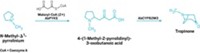
Now, Thomas Wirth at Cardiff University and his colleagues have developed a short and efficient ajoene synthesis (Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201808605). Wirth and his team were hoping to find a better way to produce large quantities of ajoene so they could test it and its analogs as potential drug candidates.
Working with easily available starting materials, the team began on a milligram scale to optimize the reaction. They devised a pathway that first introduced a selenium moiety to a dibromide molecule. That selenium moiety stayed in place until the final step, where it was used to introduce an alkene as the selenium was eliminated. In the final step of the synthesis, two transformations happened at once: Hydrogen peroxide both removed the selenium and oxidized a sulfide group to sulfoxide.
Eric Block, an expert on the chemistry of garlic compounds at the University at Albany not involved with the work, says that Wirth’s recipe is “a clever and efficient synthesis,” and the final double-pronged step particularly so.
That final step, which introduces two different functionalities at the same time, was the trickiest part of the process, Wirth says. “It did work as planned,” he explains, “but during purification of this last step some of the product was lost.”
When the team scaled up their synthesis, however, that last transformation became easier. The yield of the final step in the ajoene synthesis went from 27% on the original milligram scale to 65% on a hundred-gram scale.
On a larger scale, it’s easier to add the hydrogen peroxide carefully in small amounts, Wirth says, which could explain the improvement. The researchers made 169 g of ajoene—which retains that distinctive pungent garlicky smell.
The team also compared how well their synthetic ajoene worked compared to ajoene extracted from garlic. They chose to look at ajoene’s ability to inhibit quorum sensing, which bacterial cells use to communicate. The synthetic ajoene was able to inhibit quorum sensing in a biofilm just as well as the garlic-derived ajoene.
The team included colleagues from Neem Biotech, a Welsh company working on antimicrobials. Now that ajoene is easy to make on a large scale, studying its biological activity as well as those of its analogs will be much easier, Block says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter