Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Natural Products
A natural product that finishes its own synthesis
Plant pathway to potential obesity compound concludes with nonenzymatic conversions
by Laurel Oldach
June 30, 2023

Plants produce many pharmaceutically interesting compounds—but it can be hard to determine how they do it. Their metabolic pathways are complicated, with many by-products and dead ends. Researchers at the University of Copenhagen report in Nature Chemistry on the biosynthetic pathway of a molecule called celastrol, finding to their surprise that the last few synthetic steps can occur spontaneously (2023, DOI: 10.1038/s41557-023-01245-7).

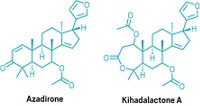
Celastrol, a terpenoid from roots of a vine used for traditional Chinese medicine, was reported to combat weight gain in overfed mice in 2015 (Cell 2015, DOI: 10.1016/j.cell.2015.05.011). The finding ignited interest in using celastrol to treat obesity, but it is difficult to synthesize efficiently, and isolating it from roots can yield varying amounts and undesirable copurifying compounds.
Researchers led by Sotirios Kampranis, Søren Bak and Karel Miettinen investigated how the vine synthesizes celastrol, focusing on metabolic enzymes in the cytochrome P450 family, or CYP450s. From 228 candidate CYP450s in the vine’s genome, they identified 21 expressed in the roots and transferred these enzymes into tobacco and yeast. They used liquid chromatography and mass spectrometry to test for celastrol and structurally characterize intermediates, delineating a pathway to celastrol that was different than the field had predicted, in which 3 CYP450s produce the first 7 conversions.
“I continue to be amazed by the scope of reactions that P450s can catalyze, even after 50 years in this field,” writes F. Peter Guengerich of Vanderbilt University in an email calling the new study a tour de force.
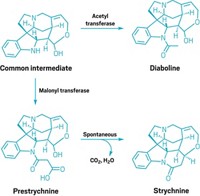
Surprisingly, the researchers found that after the CYP450s’ work was done, the final synthetic steps required no help from an enzyme. While many biosynthetic pathways include spontaneous reactions, Kampranis says, “it’s usually one or two steps, no more.” In contrast, an isolated celastrol precursor can undergo 9 reactions including decarboxylation, oxidation, aromatization and isomerization in succession in a test tube, and the team could not find an enzyme that accelerates the process.
The researchers have applied for a patent on celastrol production with a combination of engineered yeast and chemical synthesis, which may be useful in determining whether the compound does have medicinal value. They say they suspect that cascades of spontaneous reactions could help solve other natural product synthetic puzzles. But Guengerich isn’t so sure that the spontaneous reactions they observed can occur fast enough in the plant root to make a significant amount of celastrol. Though the researchers did not find an enzyme involved in the conversions, they did not rule one out, he says. “Time will tell if there is a catalyst or not in the cells.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter