Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthetic Biology
Discovery of new poppy enzyme might help improve yeast-based opiate synthesis
Adding enzyme to engineered yeast boosts production of thebaine, a key morphine intermediate
by Celia Henry Arnaud
May 31, 2018
| A version of this story appeared in
Volume 96, Issue 23

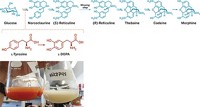
Researchers want to engineer yeast to synthesize various opiates instead of depending on opium poppies to generate them. Poppy farming is a dangerous business that can lead to illicit drug sales and risks losing key materials to crop failure. To engineer the yeast, though, scientists need to find all the enzymes that poppies coordinate to make opiates.
One of those opiates is thebaine, an intermediate along the biochemical pathway that makes codeine and morphine. Thebaine is also a starting material in the industrial semisynthesis of drugs such as the pain medication oxycodone and the addiction treatment naltrexone.
Three years ago, researchers thought they finally knew the entire pathway poppies use to produce thebaine. They posited that the final step in that pathway—an allylic rearrangement of (7S)-salutaridinol 7-O-acetate to form thebaine—occurs spontaneously. For that rearrangement to occur spontaneously, however, it must occur at pH 8–9. Under natural conditions, closer to pH 7, the process occurs, but it’s very inefficient.
Now Peter Facchini, a biology professor at the University of Calgary and chief scientific officer of the start-up Epimeron, and coworkers have identified a previously unknown enzyme in poppies that catalyzes the final step efficiently (Nat. Chem. Biol. 2018, DOI: 10.1038/s41589-018-0059-7). They’ve dubbed the enzyme thebaine synthase.
Scientists had already shown that yeast engineered with the entire poppy biochemical pathway but without the newly identified synthase could make thebaine, but only in very small amounts. Under natural pH conditions, the modified microbes also generated an unstable hydroxylated by-product that was favored over thebaine.
Facchini’s team suspected poppies must have an enzyme that favors producing thebaine instead of the by-product. The researchers used protein chromatography to isolate candidates for the enzyme.
Vincent Martin of Concordia University has also been working on engineering yeast to produce opiates, and his group had also found the final step in thebaine synthesis to be limiting. “Thebaine synthase will be an important enzyme for those interested in producing thebaine and its derivatives in yeast,” Martin says. “But even with this enzyme, we are still far from achieving commercially relevant levels of these compounds in yeast.”
In collaboration with scientists at Epimeron, including Facchini, bioengineers at the biotech firm Intrexon engineered yeast with the poppy pathway containing the gene for thebaine synthase. Without any optimization, the engineered microbes produced 24-fold more thebaine than engineered yeast with the poppy pathway that relied on spontaneous conversion during the last synthetic step.
“When one additional gene in your first experiment can make a 24-fold improvement, you know you’re on your way,” Facchini says. He adds that he’s not done looking for genes that can help clear other bottlenecks in the biosynthesis of thebaine.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter