Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Tissue Engineering
Sex hormones’ effect on cultured cells depends on chromosomal background
Widely used media ingredient may confound primary culture studies, Tulane researcher says
by Laurel Oldach
April 4, 2024
| A version of this story appeared in
Volume 102, Issue 11

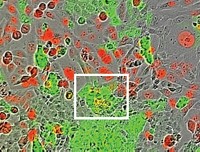
Researchers at Tulane University have found that whether cells are derived from male or female donors can affect how they grow in common cell culture media. They say the study could help explain why a proportion of cell culture experiments are difficult to reproduce.
Whether studying the chemistry of life or testing the potential—and potential hazards—of drug candidates, many scientists use cell culture models to mimic humans as closely as possible. Emerging technologies for culturing cells isolated from humans, like organoids and organ-on-a-chip models, grow cells in layers or matrices to resemble their tissue context.

In a presentation at ACS Spring 2024 and a preprint (a study that has yet to be peer-reviewed), Tulane biomedical engineer Mark Mondrinos and colleagues report that the responses of primary cells to the human sex hormones estradiol and dihydrotestosterone depend on the sex chromosomes of the person they were isolated from (bioRxiv 2023, DOI: 10.1101/2023.07.23.550236).
To study the effect of hormones in their model of blood cell growth, the researchers first had to modify the fetal bovine serum they used in growth media. The serum, widely used for cell culture, is rich in estrogens that may have confounded results. After using activated charcoal to remove those estrogens and adding back a known quantity of hormones, the team could see that primary cells from people with two X chromosomes multiply and form new blood vessels best in media that contains estradiol. Cells from people with both an X and a Y chromosome fare better in media that’s rich in testosterone. The researchers also observed that this interaction between chromosome background and hormones affected cells’ responses to other stimuli, like an inflammatory stimulus.
“This could be a huge source of variability” in studies of primary cells in culture, Mondrinos says. He thinks that growing cells in media that better match the growing environment in the body they come from may improve cell culture efforts in biotechnology and help improve the understanding of the biological mechanisms behind sex differences in a variety of diseases.
Deborah Clegg, whose lab studied the effects of hormones on metabolism before she became vice president for research at Texas Tech University, says the preprint sheds light on a pervasive oversight in research. Almost no studies report on the chromosomal sex of the cells they use, and few consider the way hormones in serum may affect results. Scientists “really need to pay attention to the hormonal milieu of the cell culture,” Clegg says.
Mondrinos is following up with transcriptomic studies to understand signaling differences between XX and XY cells. He says he suspects that many primary cells will show sex-dependent responses to hormones, which could affect many different tissue culture models. The phenomenon may have gone unnoticed because researchers have historically cultured cancer cell lines that can replicate indefinitely. “Finer aspects of cell physiology, like differences in hormone receptor expression and attachment to other pathways . . . that stuff kind of goes out the window a little bit once the cells become transformed and aberrant,” Mondrinos says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter