Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Products
What’s that Stuff
What are glow sticks, and what’s the chemical reaction that makes them light up?
Mixing hydrogen peroxide, oxalate esters, and certain dyes results in some illuminating chemistry
by Bethany Halford
October 19, 2021
| A version of this story appeared in
Volume 99, Issue 39
Breaking something rarely sparks joy—unless you’re activating a glow stick. Just bend the plastic baton until you hear a snap, and behold, you have a radiant wand to illuminate your way. Whether you’re trick-or-treating on Halloween or dancing the night away, glow sticks provide a cool source of light. That light is made by a chemical reaction—a phenomenon known as chemiluminescence.
In glow sticks, the chemicals that react together to create the light are kept separate until the right moment. The glow stick’s outer plastic tube holds a solution of an oxalate ester and an electron-rich dye along with a glass vial filled with a hydrogen peroxide solution. The signature snap that starts the reaction signals that you’ve broken the glass tube, releasing the hydrogen peroxide. When the chemicals mix, the reaction goes through several steps before releasing light.
First, the hydrogen peroxide and oxalate ester react to form a high-energy intermediate, but the precise nature of that intermediate is still something of a mystery, says Gary B. Schuster, a chemistry professor at the Georgia Institute of Technology who has studied the reaction. Many chemists believe it’s the strained molecule 1,2-dioxetanedione. But despite 50 years of looking for it, there is no direct evidence of that compound, he says.
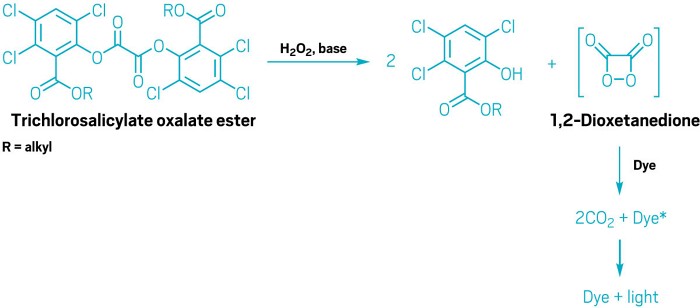
Even though chemists aren’t certain of the precise structure of the high-energy intermediate, they know it’s a good electron acceptor. It snags an electron from the dye and then breaks down into carbon dioxide and a negatively charged carbon dioxide radical anion. The dye, which has become a positively charged radical cation, then takes back an electron from the CO2 radical anion.
In taking back the electron, the dye gains excess energy. The molecule uses that energy to move into an excited state before dropping back down and emitting the energy as a photon of light. Your glow stick glows.
Although several innovations brought glow sticks to the masses, the underlying chemistry was discovered in a janitor’s closet in New Jersey in 1962. Back then, Edwin A. Chandross, a newly minted chemistry PhD working at Bell Laboratories, was tinkering with light-producing chemical reactions. “The company had very generously provided me with a dark room to do chemiluminescence experiments,” Chandross quips, alluding to the small, dark closet next to his lab.
One day Chandross exposed a xanthone derivative he’d prepared with oxalyl chloride to hydrogen peroxide with a little added anthracene dye in the mix. The reaction produced weak light. But when Chandross tried to repeat the reaction with a cleaner sample of the xanthone derivative, the mixture stayed dark. That’s when it occurred to Chandross that it had been leftover oxalyl chloride reacting with the hydrogen peroxide and dye in the first experiment. When he tried mixing those three components, the light returned.
“The patent attorney assigned to my department declined to file a patent,” Chandross says. “And I didn’t realize how significant this really was.” Chandross published the reaction in the journal Tetrahedron Letters (1963, DOI: 10.1016/S0040-4039(01)90712-9), and it caught the attention of Michael M. Rauhut, who was manager of exploratory research at the chemical firm American Cyanamid.
Rauhut and his colleague Laszlo J. Bollyky swapped the reactive oxalyl chloride that Chandross used for less reactive and longer-lasting oxalate esters, settling on an aromatic oxalate ester. American Cyanamid chemists also added a little salicylate base to catalyze the reaction as well as experimented with a rainbow of dyes. In modern glow sticks, rhodamine B produces a radiant red; 9,10-bis(phenylethynyl)anthracene gives a green glow; and 9,10-diphenylanthracene lights up with a blue hue.
While most people associate glow sticks with the novelty market, they also have more serious uses. That’s why the US military financed part of the work that the American Cyanamid chemists did to develop glow stick chemistry. Chemists led by Herbert Richter at the US Naval Air Weapons Station China Lake had been developing chemiluminescent glow sticks around the same time that Chandross made his chemiluminescent discovery at Bell Labs.
Today, the government market for glow sticks is considerable, says Donald Schmidt, a senior vice president for Cyalume Technologies, a company that grew out of American Cyanamid. He says the government market is about $35 million a year compared with his estimate of about $40 million for the novelty market.
Cyalume provides glow sticks that the US military and Department of Defense use for training exercises and field operations, and glow sticks can save lives. Flotation vests equipped with the devices, for example, can help rescuers find people lost at sea. When there is a danger of flammable fumes, glow sticks can provide illumination without the risk of ignition.
In the nearly 60 years since glow stick chemistry was first discovered, there have been several chemical innovations, says Linda Jacob, Cyalume’s director of chemical services.
Several of those changes relate to safety. For example, the oxalate esters that were used in glow sticks decades ago produced trichlorophenols—chemicals that could go on to form toxic halogenated dioxins. To eliminate trichlorophenols, Jacob says, Cyalume turned to trichlorsalicylate oxylate esters, which are safer. The firm also now uses butyl benzoate and triethyl citrate as solvents instead of phthalates, some of which have been identified as endocrine disruptors. Most novelty glow sticks still use phthalates, however, she says.
Chemical innovations have also helped expand glow sticks’ lifetimes and operating temperatures. Jacob says chemists have learned how to tune the chemiluminescent reaction’s illumination time by tweaking the salicylate catalyst. “You can change the length of the chemiluminescence from 30 seconds to 24 hours,” she says. Other chemical additives allow glow sticks made for the military to work whether the temperature is –50 or 50 °C. “Whether you’re in the Arctic or in the desert in the hot summer sun, you’ll get pretty close to the same performance,” Jacob says.
So the next time you snap a glow stick, take a moment to appreciate the cool chemistry that brightens your night.
UPDATE
This story is an update to the What’s That Stuff? article on light sticks that was published on Jan. 18, 1999, and written by Elizabeth Wilson. [https://pubsapp.acs.org/cen/whatstuff/stuff/7703scit4.html] This article has new details about chemicals in glow sticks.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter